Antigen-binding molecule capable of binding to plurality of antigen molecules repeatedly
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
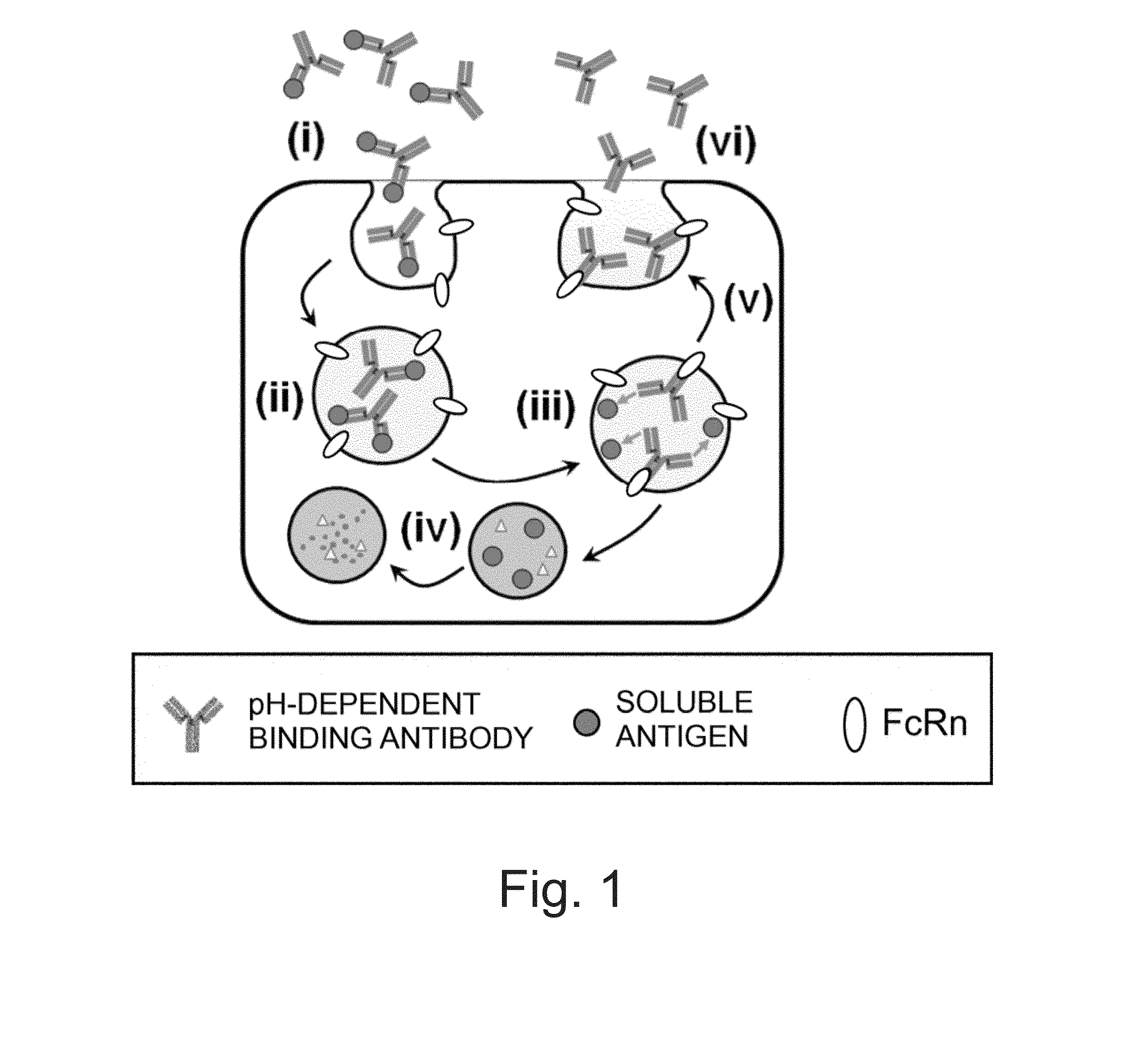
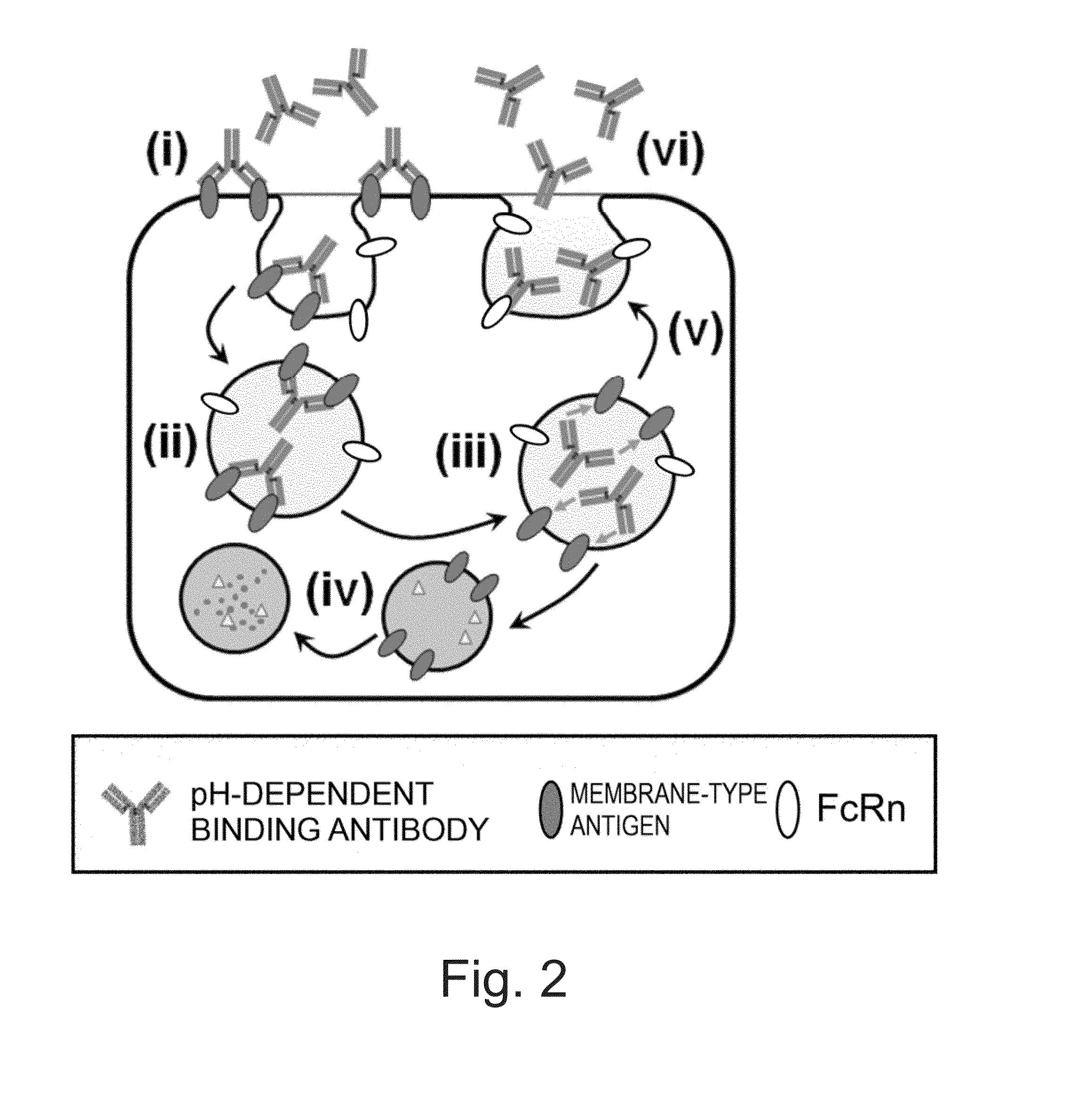
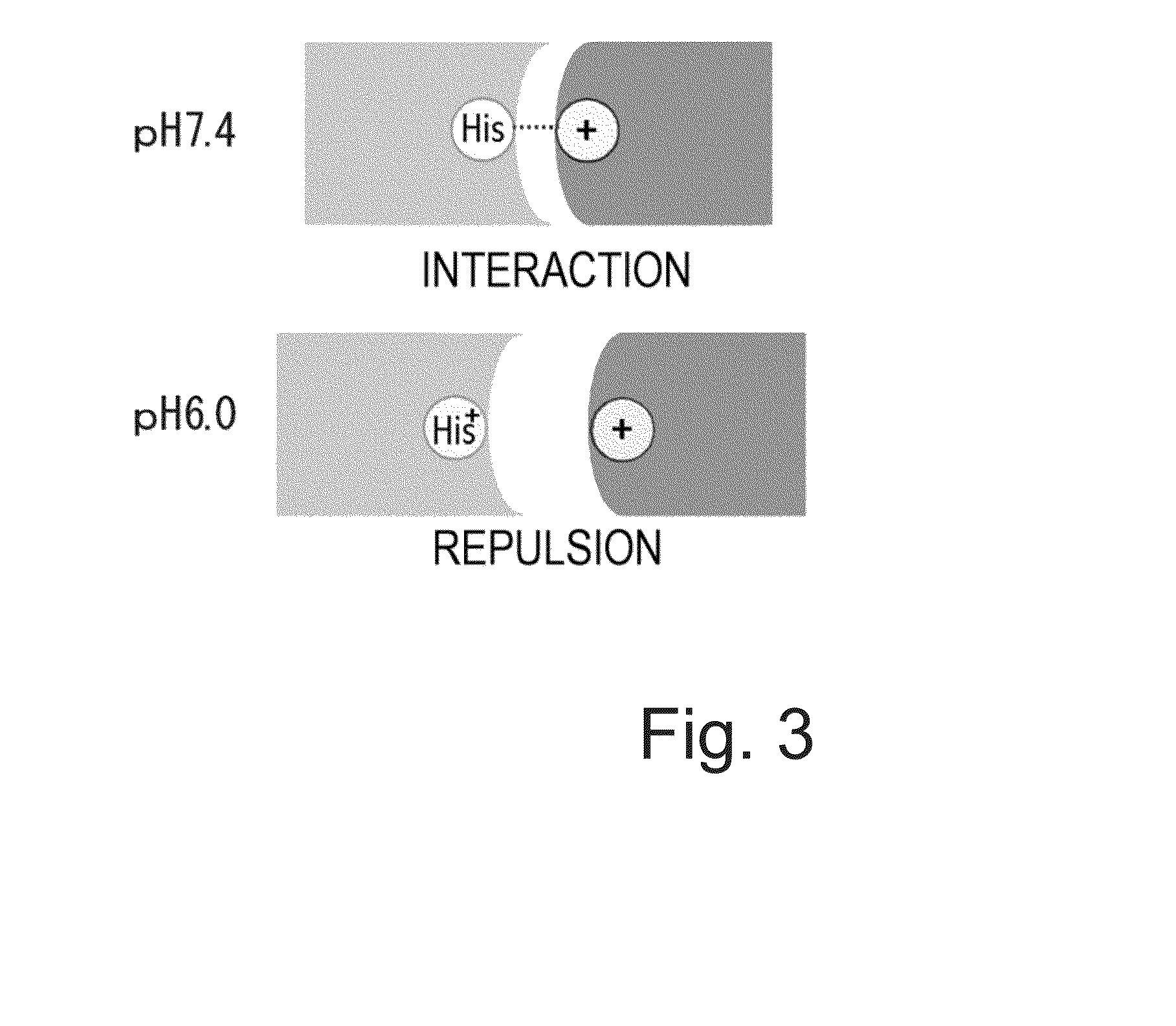
The Concept of Antigen Elimination-Accelerating Effect of Calcium-Dependent Antigen-Binding Antibodies
[0385](1-1) Effect of pH-Dependent Antigen-Binding Antibodies to Accelerate Antigen Elimination
[0386]H54 / L28-IgG1 described in WO 2009 / 125825 is a humanized anti-IL-6 receptor antibody. Fv-4-IgG1 is a humanized anti-IL-6 receptor antibody that results from conferring H54 / L28-IgG1 with the property to bind to soluble human IL-6 receptor in a pH-dependent manner (which binds under neutral condition but is dissociated under acidic condition). The in vivo test described in WO 2009 / 125825 using mice demonstrated that the elimination of soluble human IL-6 receptor could be greatly accelerated in a group administered with a mixture of Fv-4-IgG1 and soluble human IL-6 receptor as antigen as compared to a group administered with a mixture of H54 / L28-IgG1 and soluble human IL-6 receptor as antigen.
[0387]Soluble human IL-6 receptor bound to a general antibody that binds to soluble human IL-6 r...
example 2
Isolation of Ca-Dependent Binding Antibodies from Human Antibody Library Using Phage-Display Technique
(2-1) Preparation of Phage-Display Library of Naive Human Antibodies
[0393]Several human antibody phage-display libraries that present Fab domains comprising a human antibody sequence were constructed using as a template polyA-RNA prepared from human PBMC, human polyA RNA available on the market, or the like, according to Methods Mol. Biol. 2002, 178: 87-100.
(2-2) Isolation of Ca-Dependent Binding Antibody Fragments from Libraries by Bead Panning
[0394]The first selection from constructed human antibody phage-display libraries was achieved by enriching antibody fragments having antibody-binding ability or by enriching using the Ca-dependent binding ability as an indicator. Antibody fragments with a Ca-dependent binding ability were enriched by eluting phages via EDTA chelation of Ca ion after antibody fragments were bound to an antigen in the presence of Ca ion. Biotinylated human IL-...
example 3
Assessment of the Prepared Antibodies for their Ca-Dependent Binding Activity to Human IL-6 Receptor
[0401]Antibodies 6RL#9-IgG1 (heavy chain SEQ ID NO: 1; light chain SEQ ID NO: 2), 6RK#12-IgG1 (heavy chain SEQ ID NO: 66; light chain SEQ ID NO: 67), and FH4-IgG1 (heavy chain SEQ ID NO: 3; light chain SEQ ID NO: 4) prepared in Example 2 were assessed for their binding activity to human interleukin 6 receptor (hIL6R) at pH 7.4 using Biacore T100 (GE Healthcare). The assay was carried out using as a running buffer 0.05% Surfactant P20, 10 mmol / l ACES, 150 mmol / l NaCl (pH 7.4 or 6.0) containing 3 μM or 2 mM CaCl2.
[0402]After immobilizing an adequate amount of recombinant Protein A (Thermo Scientific) onto Sensor chip CM4 (GE Healthcare) by an amino coupling method, antibodies were allowed to bind onto the sensor chip. An appropriate concentration of hIL-6R was injected as an analyte to interact with antibodies on the sensor chip. Then, 10 mmol / l glycine-HCl (pH 1.5) was injected to rege...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com