Targeted Lipid-Drug Formulations for Delivery of Drugs to Myeloid and Lymphoid Immune Cells
a technology of myeloid and lymphoid immune cells and drug delivery system, applied in the field of medical arts, can solve the problems of inability to achieve human-practical strategy, inability to develop long-circulating large (>500 nm) liposomes using steric stabilization methods, and inability to achieve drug delivery. to achieve the effect of facilitating production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Materials and Methods
[0096]Preparation of Liposomes.
[0097]A 30 μmol lipid film composed of DOPC / Chol / DOPE-MBP (36.5:33.0:2.5 mol:mol:mol) was formed (cholesterol was purchased from Calbiochem, San Diego, Calif., USA; and DOPE and DOPE-MPB were from Avanti Polar Lipids, Alabaster, Ala., USA). Lipid films were hydrated with 1 ml 50 mM calcein (Molecular Probes, Eugene, Oreg., USA) in PBS (pH 7M), sonicated in a bath sonicator (5 min) and extruded ×5 through a 0.1 μm nucleopore filter (Avanti Polar Lipids) using a hand-held extruder. Also, freeze-thaw cycles can be employed. The mean liposome size was determined by quasielectric light scattering with a Nicomp 380 ZLS Zeta-Potential Particle Sizer (Particle Sizing Systems, Santa Barbara, Calif., USA), yielding an average diameter of 146.7±31.0 nm.
[0098]Protein A Liposomes.
[0099]To be able to test the targeting ability of different antibodies with a standardized liposome, immunoglobulin-molecules were coupled to liposomes via protein A o...
example 2
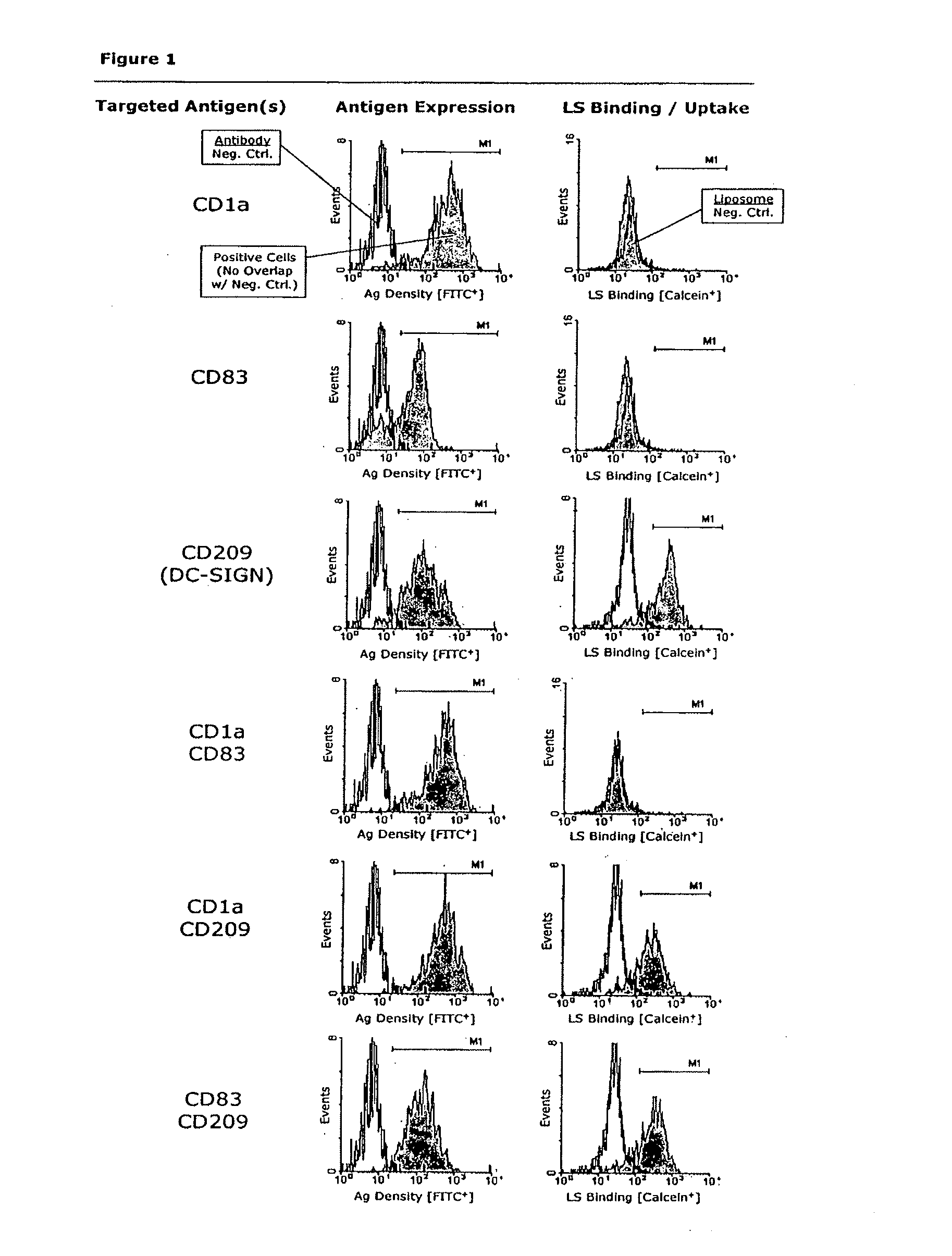
Active Targeting of Immune Cells with Monospecific or Bispecific Immunoliposomes
[0142]Peripheral blood mononuclear cells (PBMNCs) were evaluated according to their size (forward scatter) and granularity (side scatter) and thus were gated as naïve T and B cells; activated T-cells and B-cells; and monocytes, including a small proportion of blood dendritic cells (data not shown). Cultured monocyte-derived dendritic cells (MoDCs) were tested for expression of markers delineating their developmental stage (maturity), as well as for DC subtype markers. The DCs expressed markers typical for skin and mucosal DC phenotypes that are considered to play a key role in HIV infection. When being infected via the mucosal route, mucosal DCs are the first immune cell type to be directly infected by HIV (and integrate its genetic information as proviral DNA) and / or harvest HIV on their surface by DC-SIGN and / or take up HIV by any of various mechanisms to retain it in intracytoplasmic compartments (e.g...
example 3
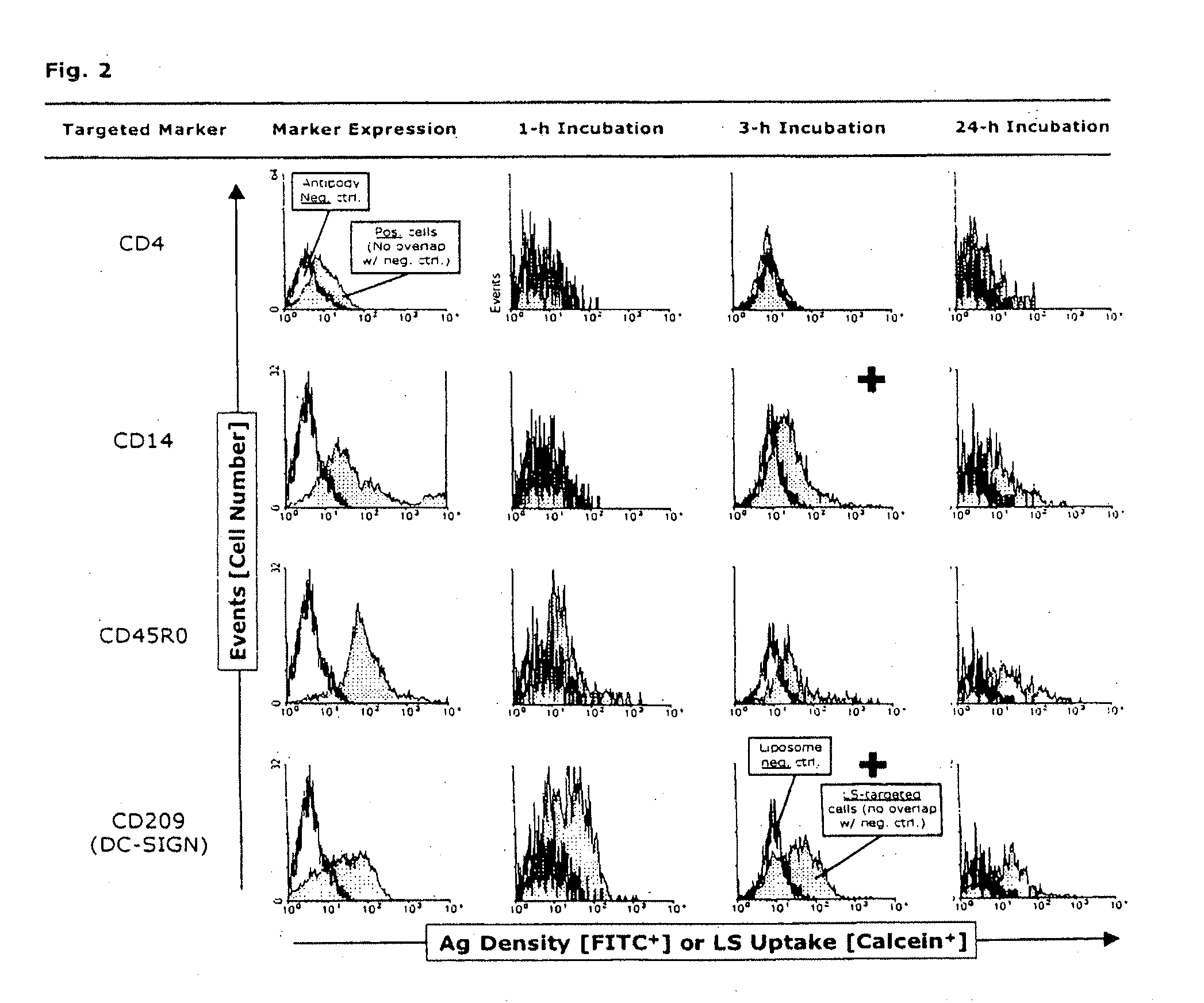
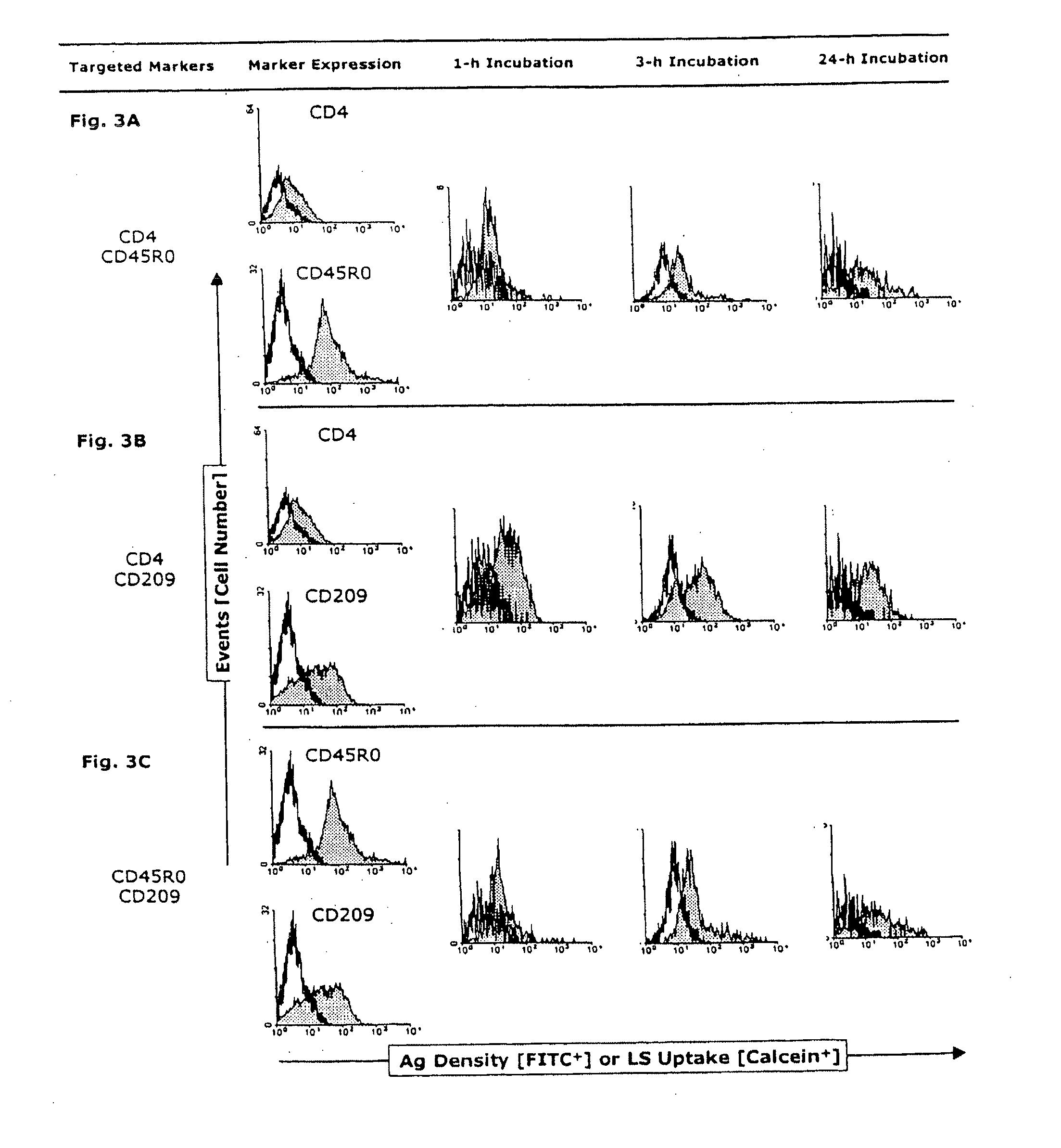
Fluorescence-Microscopic Uptake Studies
[0158]After infection with HIV-1, intracytoplasmic compartments with accumulated infectious virus are demonstrable in both immature and mature MyDCs (Frank, I et al., Infectious and whole inactivated simian immunodeficiency viruses interact similarly with primate dendritic cells (DCs): differential intracellular fate of virions in mature and immature DCs, J Virol 76:2936-51 [2002]). Therefore for comparison, immature or mature MoDCs were incubated for 3, 4 or 5 h at 37° C. with anti-CD209-labeled liposomes (each at n=3). The cells were then harvested as described above and gently pipetted to dissociate homotypic clusters (as controlled by phase microscopy). Pelleted single cells were successively dissolved in 100 μl of ProLong antifade mounting medium to which was added 5 μM of the positively charged AT-binding DNA dye, 4′,6-diamidino-2-phenylindole (DAPI) (both from Molecular Probes, Eugene, Oreg., USA). Fifty μl of each preparation were trans...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com