Method for producing hexagonal boron nitride film using borazine oligomer as a precursor
a technology of boron nitride and borazine oligomer, which is applied in the direction of plasma technique, metal/metal-oxide/metal-hydroxide catalyst, etc., can solve the problems of different number of layers, limited size and thickness control of hexagonal boron nitride exfoliated by chemical exfoliation, etc., and achieves low cost and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
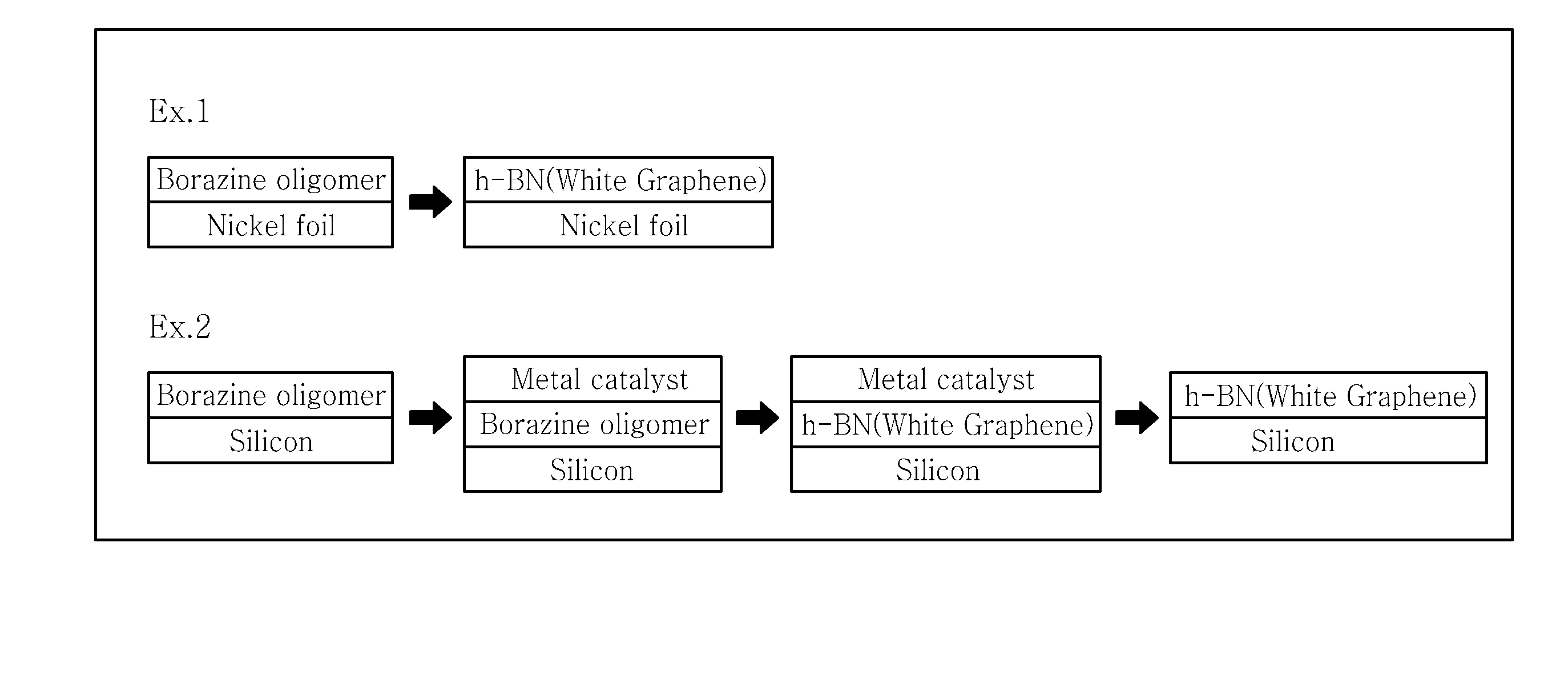
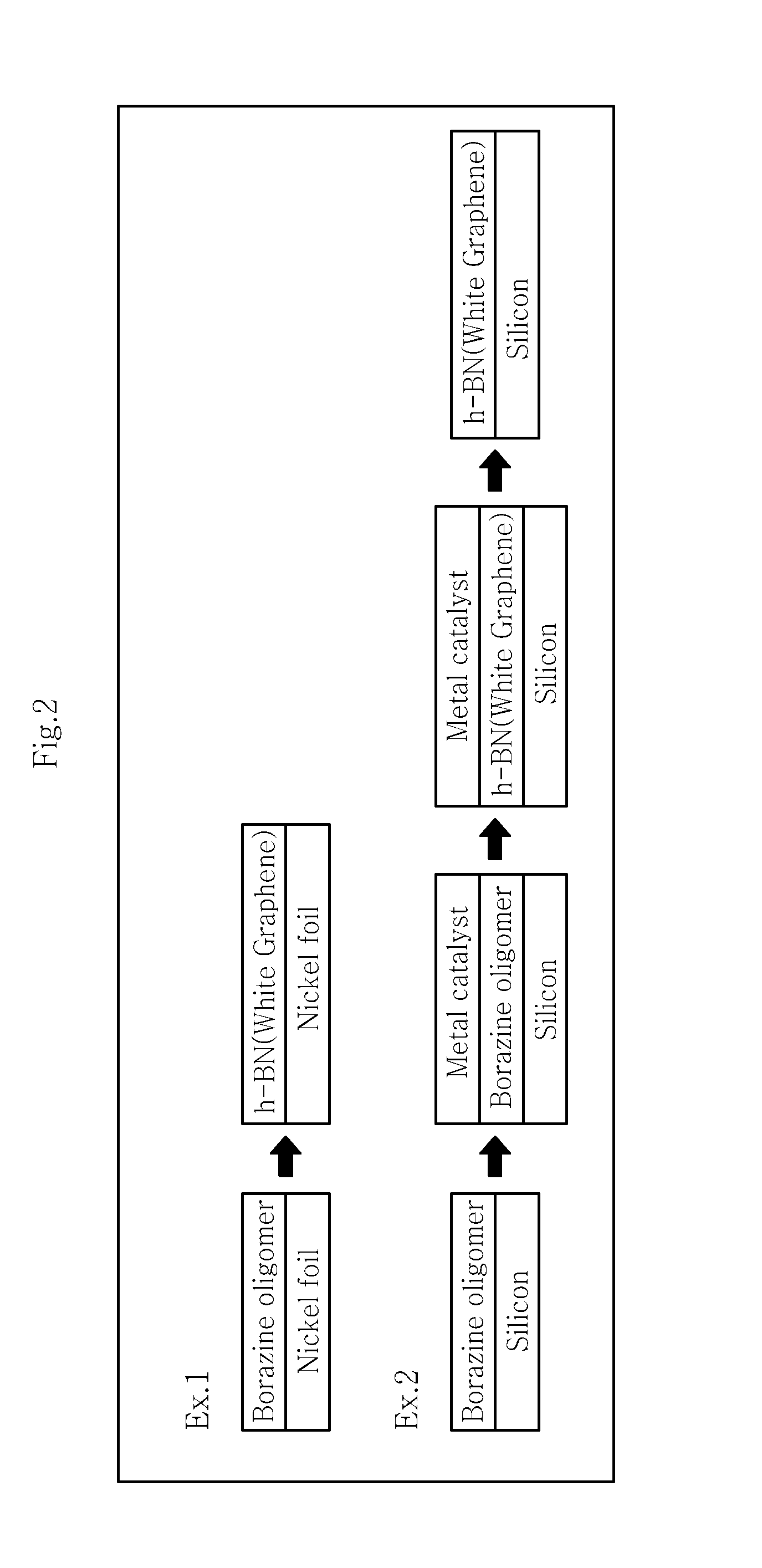
example 1
[0103]A solubilized borazine oligomer (boron nitride precursor solution) is used to prepare a hexagonal boron nitride film. For this, a borazine oligomer ((B3H3H4)x) is prepared through the thermal oligomerization of borazine.
[0104]Next, the borazine oligomer is mixed with chlorobenzene to obtain a boron nitride precursor solution optimized for coating.
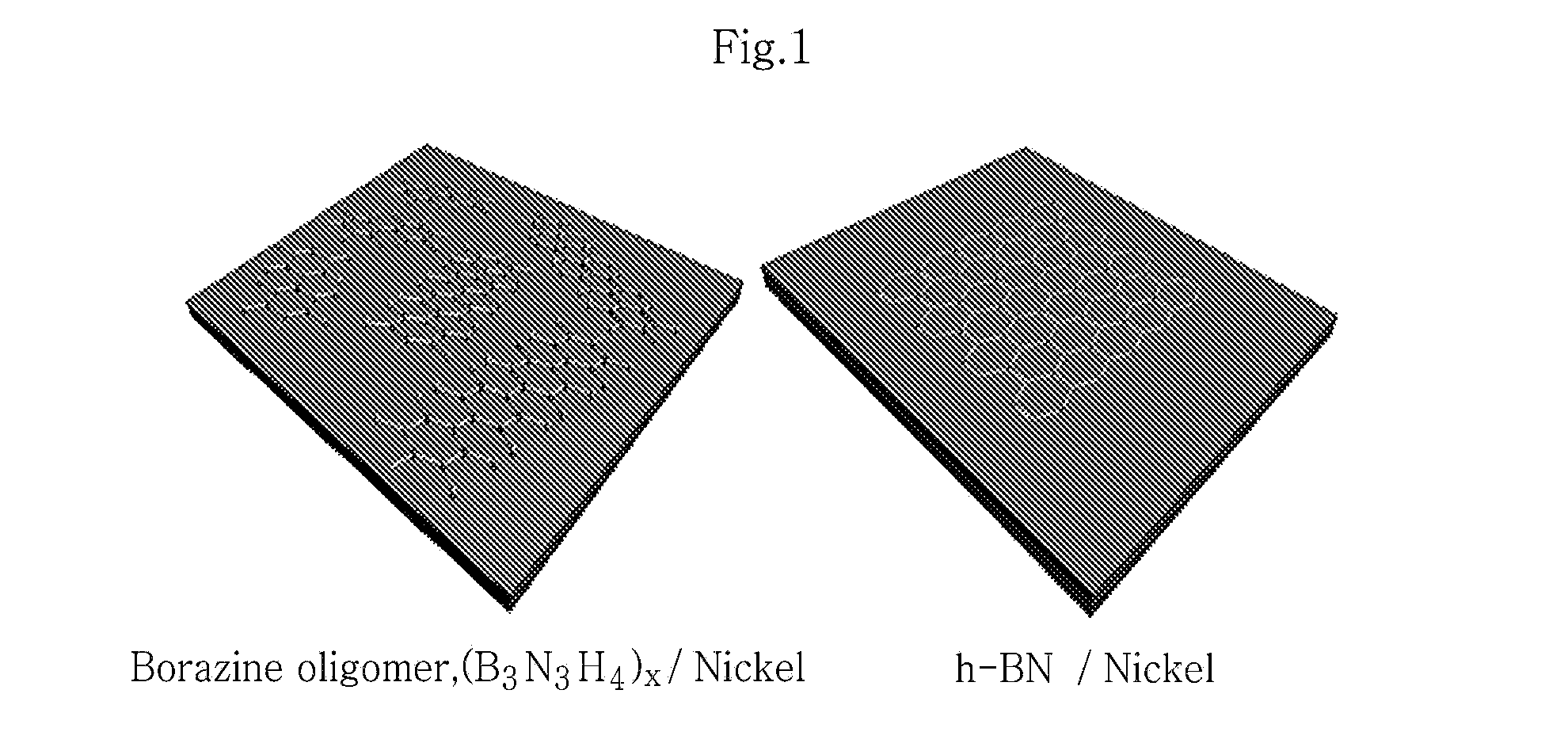
[0105]As a metal catalyst substrate, nickel foil is provided. The nickel foil is treated by electrochemical polishing under the condition of 2 volt for 20 minutes. Then, metal surface treatment is carried out through a 5-minute cleaning process including introduction into acetone and ethanol, followed by sonication.
[0106]The solubilized borazine oligomer is coated onto the metal catalyst substrate through spin-coating under the condition of 9000 rpm. Then, baking is carried out at 150° C. for 20 minutes.
[0107]Then, the borazine oligomer coated on the metal catalyst substrate is subjected to heat treatment by increasing the temperature...
example 2
[0108]A solubilized borazine oligomer (boron nitride precursor solution) is used to prepare a hexagonal boron nitride film. For this, a borazine oligomer ((B3H3H4)x) is prepared through the thermal oligomerization of borazine.
[0109]Next, the borazine oligomer is mixed with chlorobenzene to obtain a boron nitride precursor solution optimized for coating.
[0110]The solubilized borazine oligomer is coated onto a silicon substrate through spin-coating under the condition of 9000 rpm. Then, baking is carried out at 150° C. for 20 minutes.
[0111]Then, a nickel metal catalyst is deposited on the borazine oligomer coated on the silicon substrate trough electron beam evaporation.
[0112]Then, the borazine oligomer coated on the metal catalyst substrate is subjected to heat treatment by increasing the temperature to 1000° C. and carrying out heat treatment under argon (Ar) atmosphere at a pressure of 600 mtorr for 1 hour, followed by cooling for 2 hours.
[0113]Example 1 and Example 2 are illustrat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com