Antibodies targeted to fungal cell wall polysaccharides
a technology of fungal cell wall and antibodies, which is applied in the direction of antibody medical ingredients, carrier-bound antigen/hapten ingredients, peptides, etc., can solve the problems of affecting the quality of life of patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
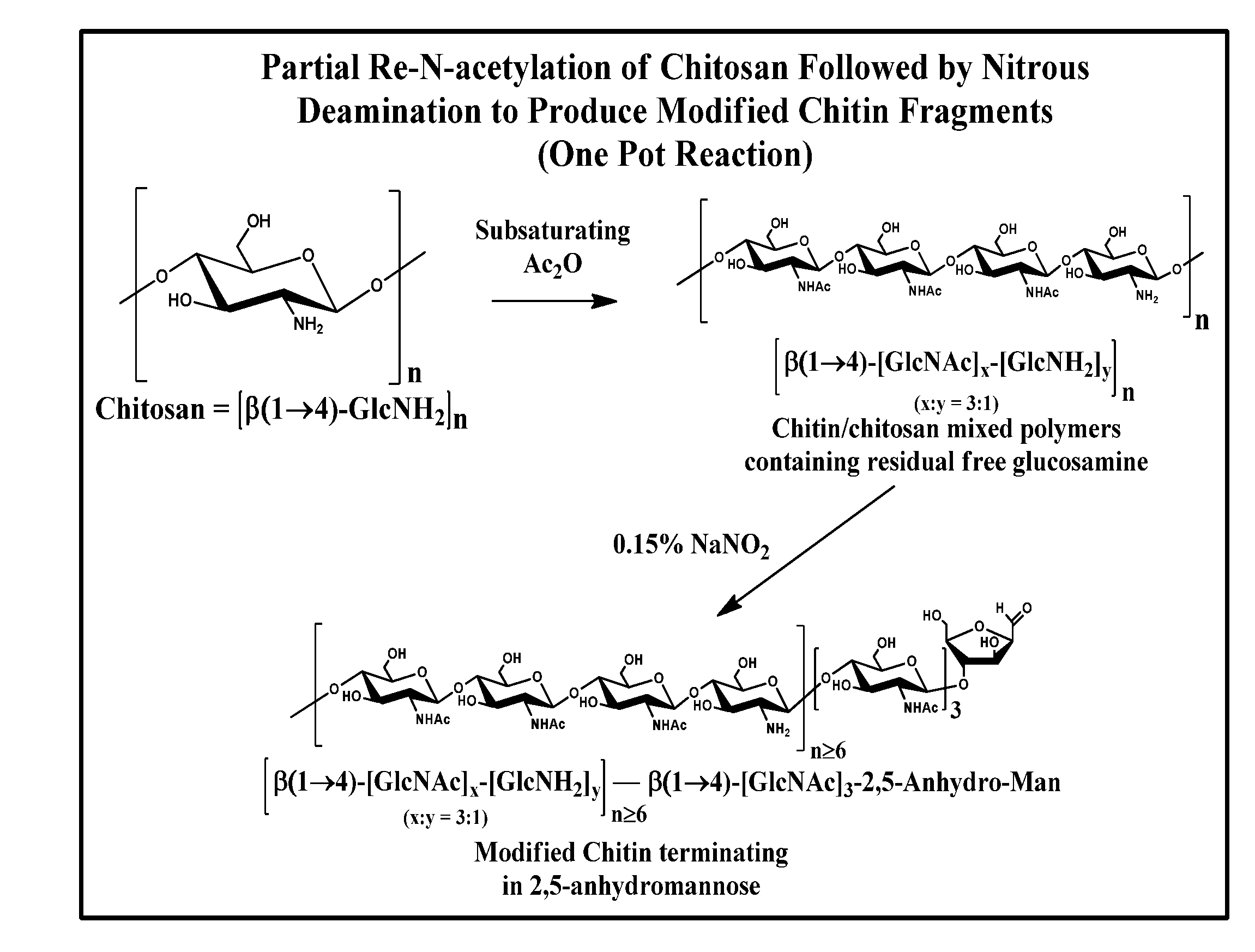
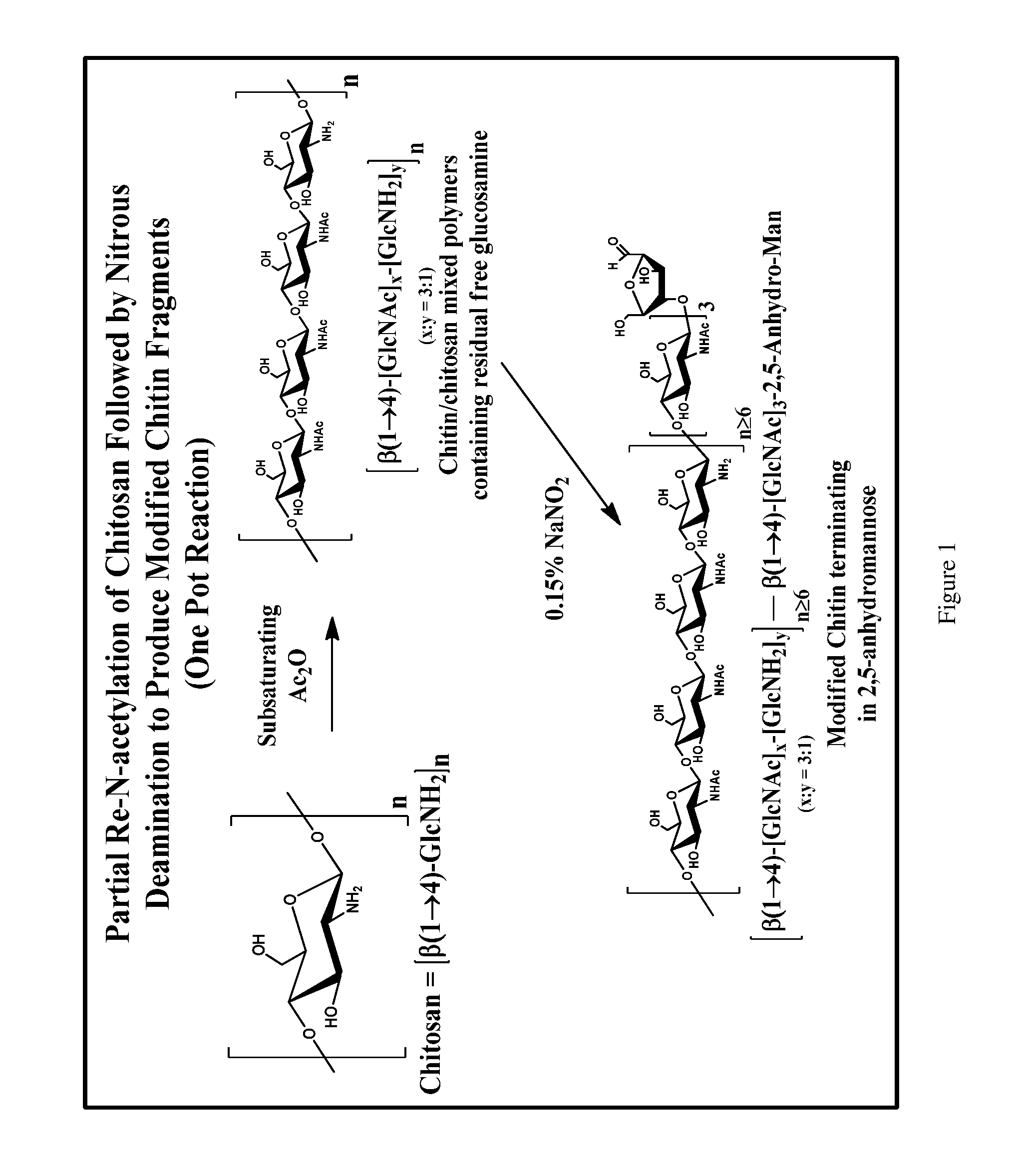
Partial Re-N-Acetylation / Deamination of Chitosan to Yield Modified Chitin Fragments
[0060]3 g of chitosan (16.8 mmol of GlcNH2; Sigma cat. #419419) was suspended in 150 mL of H2O in a 250 mL round bottomed flask and placed on a stir plate.[0061]Acetic anhydride, 795 μL, 0.5 equivalents, relative to the number of amino groups, was added to 15 mL of EtOH.[0062]The acetic anhydride solution was added to the stirring chitosan suspension, dropwise over 30 minutes, via an addition funnel. The reaction became very viscous, but it was still possible to stir the reaction mixture.[0063]The reaction was allowed to proceed for another 30 minutes at room temperature.[0064]18 mL of glacial acetic acid was added to the reaction mixture, giving a pH of ˜3.[0065]6 mL of a freshly prepared 5% aqueous solution of NaNO2 was added to the reaction and the mixture was stirred for 1 hour at room temperature. The reaction liberated a substantial amount of gas and became much less viscous during the course of...
example 2
Preparation of Modified Chitin-Tetanus Toxoid Vaccine Conjugate by Reductive Amination
[0070]5 mg of tetanus toxoid (TT, 3.3 mL from 3 mg / mL solution in saline; Sreum Staten Institute) was added to 50 mg of modified chitin fragment.[0071]The Schiff Base reaction was allowed to proceed for 6 hours at room temperature.[0072]Repurified sodium cyanoborohydride, 10 mg, was added in a volume of 10 μL H2O to the reaction mixture. The reaction was left to proceed at room temperature and monitored after one and three days by SDS-PAGE.[0073]Following the reaction, the chitin-TT mixture was diluted to ˜2 mL with PBS and dialyzed against 4 L of PBS. The dialyzed sample was filtered through a 0.45 μm syringe filter. The final dialysate was quantitated by BCA assay and analyzed by SDS-PAGE and HPLC.
example 3
Immunization of Balb / C Mice with Modified Chitin-TT Vaccine Conjugate
[0074]40 female Balb / C mice were received and housed under standard day / night cycles with food and water, ad libitum. The animals were allowed to acclimate to the facility for a minimum of one week, then randomly divided into four groups and immunized as follows:[0075]1. An emulsion of PBS in Freund's Complete Adjuvant (200 μL) was delivered by intraperitoneal injection into 10 mice on day 0. On days 28 and 38, animals received injections of PBS in Freund's Incomplete Adjuvant, delivered in the same manner.[0076]2. Modified Chitin-TT conjugate (25 μg of antigen) was injected into 10 mice as an emulsion in Freund's Complete Adjuvant (200 μL), by intraperitoneal injection on Day 0. On days 28 and 38, animals received booster injections of Chitin-TT (25 μg) in Freund's Incomplete Adjuvant, delivered in the same manner as the primary immunization.[0077]3. Animals were injected with Modified Chitin-TT (50 μg), as per th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Immunogenicity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com