Compositions and Methods for Enhancing Bioenergetic Status in Female Germ Cells
a technology of bioenergetic status and composition, applied in the field of compositions and methods for enhancing bioenergetic status of female germ cells, can solve the problems of ovaries aging and failure, decline in live birth rate, and decline in oocyte quality, so as to improve the quality of oocytes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
FACS-Based Protocol for OSC Isolation
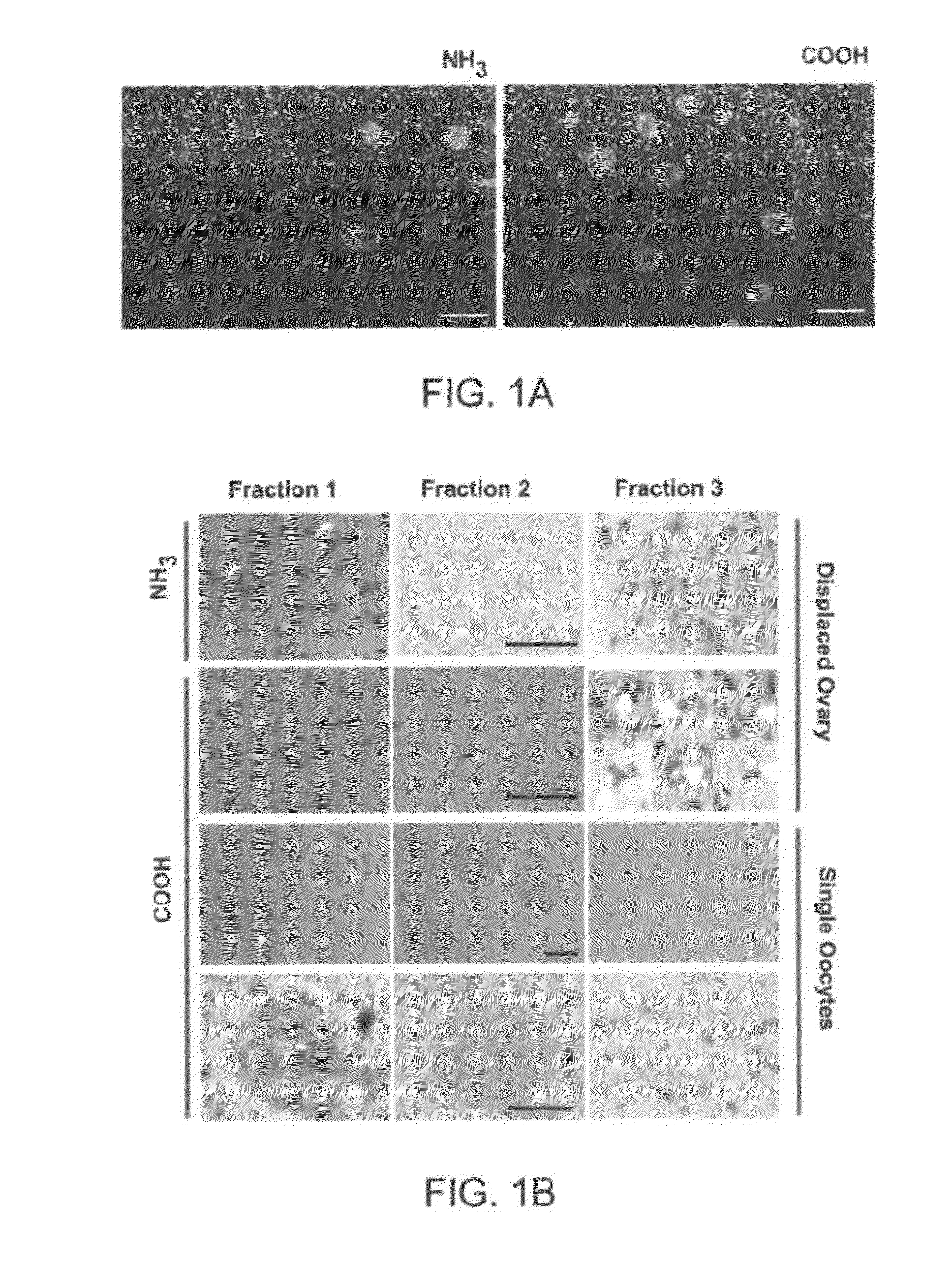
[0329]The VASA antibody used by Zou et al., Nat Cell Biol 2009 11:631-636 to isolate mouse OSCs by immunomagnetic sorting is a rabbit polyclonal against the last 25 amino acids of the COOH-terminus of human VASA (DDX4) (ab13840; Abcam, Cambridge, Mass., USA). This region shares 96% overall homology with the corresponding region of mouse VASA (MVH). For comparative studies, a goat polyclonal antibody against the first 145 amino acids of the NH2-terminus of human VASA (AF2030; R&D Systems) was used, which shares 91% overall homology with the corresponding region of mouse VASA.
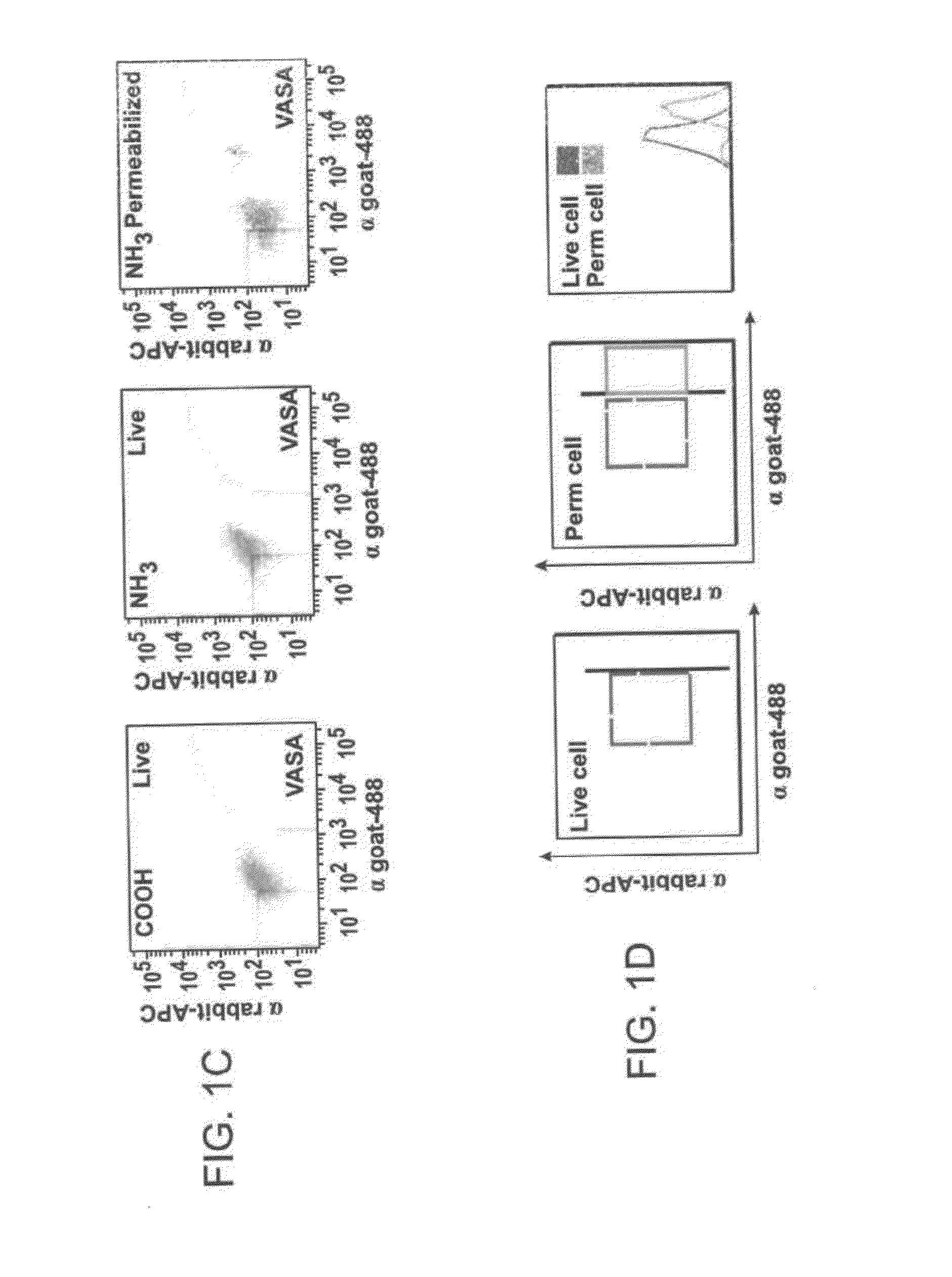
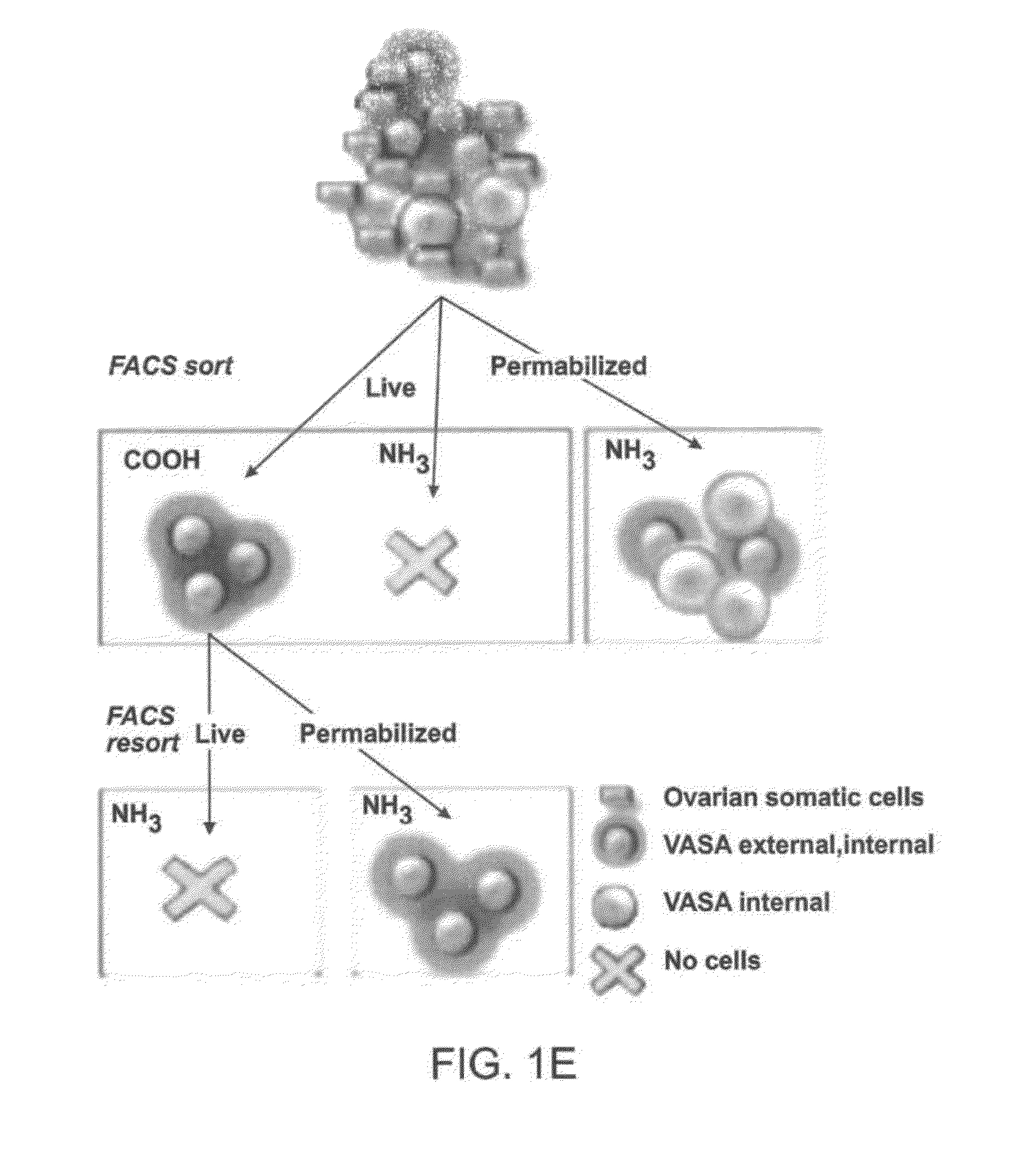
[0330]Immunofluorescence analysis of young adult (2-month-old) mouse ovaries using either antibody showed an identical pattern of VASA expression that was restricted, as expected, to oocytes (FIG. 1a). Each antibody was then used for immunomagnetic sorting of dispersed young adult mouse ovary tissue (Zou et al., Nat Cell Biol 2009 11:631-636). For each preparation of cells, ov...
example 2
Isolation of OSCs From Human Ovaries
[0334]With written informed consent, ovaries were surgically removed from 6 female patients between 22-33 (28.5±4.0) years of age with Gender Identity Disorder for sex reassignment at Saitama Medical Center. The outer cortical layer was carefully removed, vitrified and cryopreserved (Kagawa et al., Reprod. Biomed. 2009 Online 18:568-577; FIG. 12). Briefly, 1 mm-thick cortical fragments were cut into 100-mm2 (10×10 mm) pieces, incubated in an equilibration solution containing 7.5% ethylene glycol (EG) and 7.5% dimethylsulfoxide (DMSO) at 26 C for 25 minutes, and then incubated in a vitrification solution containing 20% EG, 20% DMSO and 0.5 M sucrose at 26 C for 15 minutes prior to submersion into liquid nitrogen. For experimental analysis, cryopreserved ovarian tissue was thawed using the Cryotissue Thawing Kit (Kitazato Biopharma) and processed immediately for histology, xenografting or OSC isolation. Using the COOH antibody, viable VASA-positive ...
example 3
Generation of Oocytes from FACS-Purified Mouse OSCs
[0338]The ability of FACS-purified mouse OSCs, engineered to express GFP through retroviral transduction (after their establishment as actively-dividing germ cell-only cultures in vitro) to generate oocytes following transplantation into ovaries of adult female mice was assessed. To ensure the outcomes obtained were reflective of stable integration of the transplanted cells into the ovaries and also were not complicated by pre-transplantation induced damage to the gonads, 1×104GFP-expressing mouse OSCs were injected into ovaries of non-chemotherapy conditioned wild-type recipients at 2 months of age and animals were maintained for 5-6 months prior to analysis. Between 7-8 months of age, transplanted animals were induced to ovulate with exogenous gonadotropins (a single intraperitoneal injection of PMSG (10 IU) followed by hCG (10 IU) 46-48 hours later), after which their ovaries and any oocytes released into the oviducts were collec...
PUM
| Property | Measurement | Unit |
|---|---|---|
| inner diameters | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com