Manufacture of near-net shape titanium alloy articles from metal powders by sintering with presence of atomic hydrogen
a technology of atomic hydrogen and metal powder, which is applied in the field of powder metallurgy of titanium and titanium alloys, can solve the problems of ineffective cost, inability to fully realize all the desirable advantages of titanium alloys, and ineffective cost first method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0080]A powder blend of three hydrogenated titanium powders containing different amount of hydrogen was used: (1) 25% of hydrogenated titanium powder containing 0.5 wt. % of hydrogen, particle size 2 powder containing 3.8 wt. % of hydrogen, particle size 3.
[0081]The green compact, having the thickness 12 mm, was heated to 250° C. at a slow heating rate of ˜7° C. / min and held at this temperature for 40 min to release absorbed water from the titanium powder. Then, heating was continued at the heating rate of ˜22° C. / min to a temperature in the range of 480-500° C. in the atmosphere of emitted hydrogen, and held at this temperature for 30 min to form β-phase titanium and to release reaction water from the hydrogenated titanium powders.
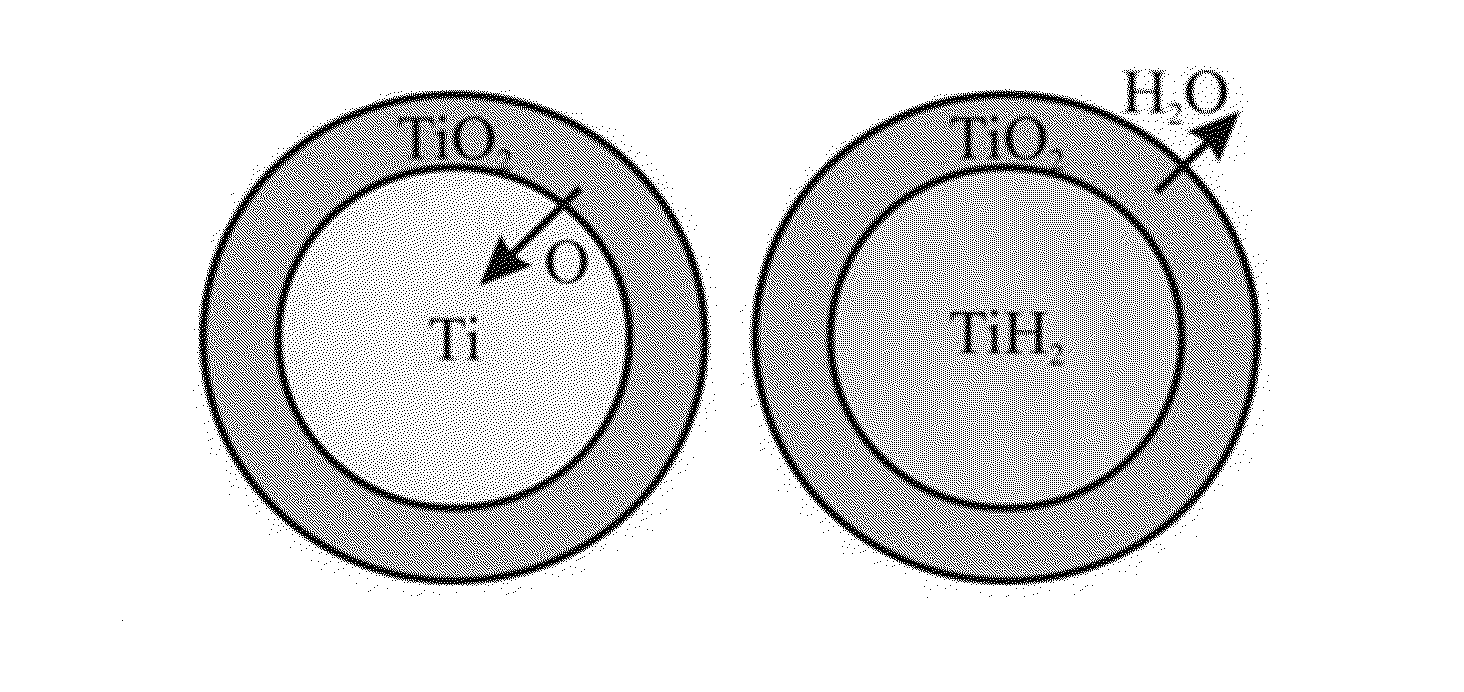
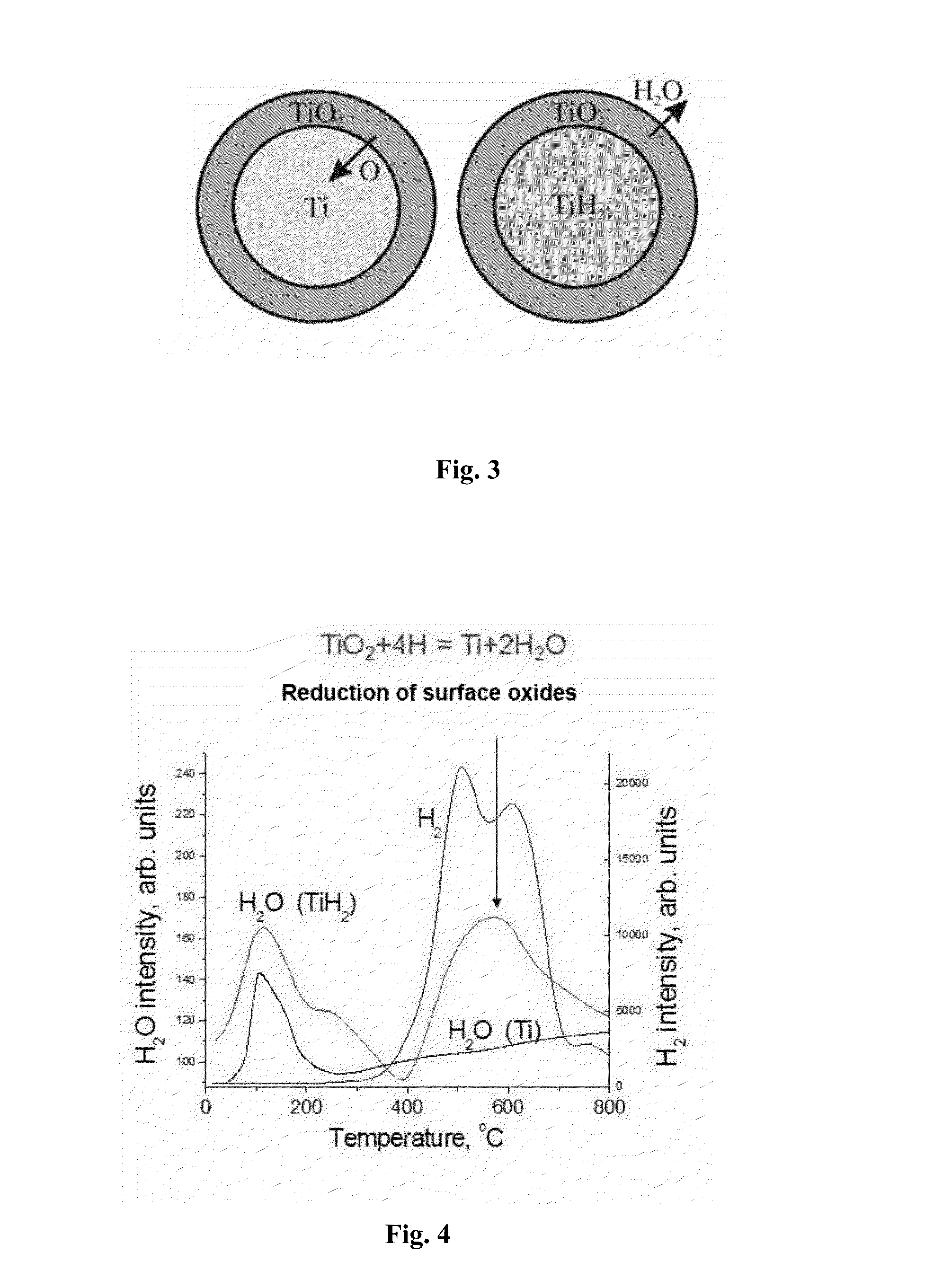
[0082]Almost complete reduction of surface oxides of the green compact particles by emitted atomic hydrogen was carried out by further heating the green compact to a temperature of 630° C. and holding at this temperature for 45 min, when the green compact...
example 2
[0093]A powder blend of two types of powders was used: (1) 20% of CP titanium powder, which does not contain hydrogen at all, particle size 2 powder containing 3.5 wt. % of hydrogen, particle size <100 microns.
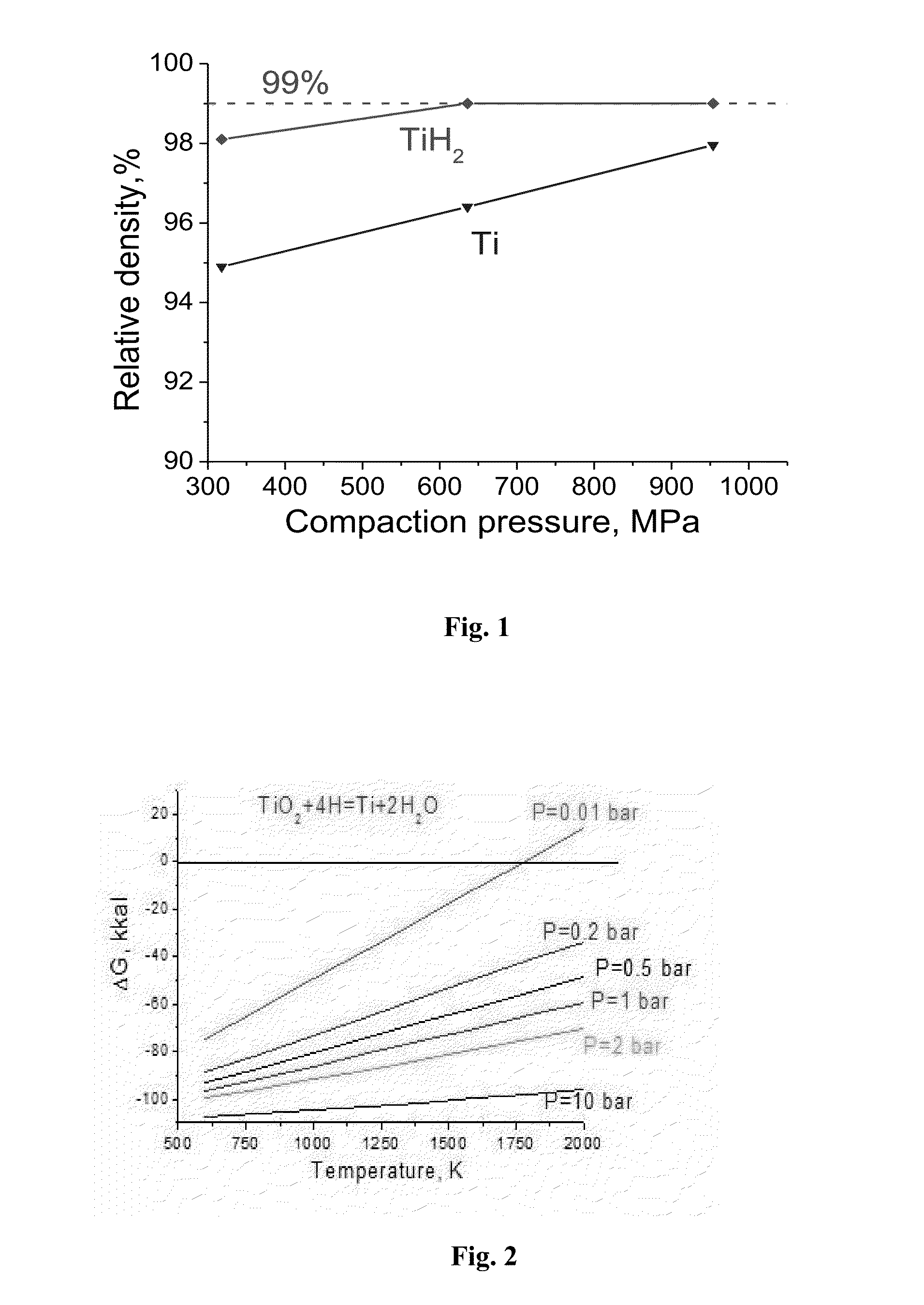
[0094]These powders were mixed together, and the obtained mixed powder was compacted at 780 MPa to a low density green compact of 3.24 g / cm3.
[0095]The green compact having the thickness 24 mm was heated to 230° C. at a slow heating rate of ˜7° C. / min and held at this temperature for 80 min to release absorbed water from the powder. Then, heating was continued at the heating rate of ˜22° C. / min to 560-580° C. in the atmosphere of emitted hydrogen and held at this temperature for 25 min to form β-phase titanium and release reaction water from the powder.
[0096]Almost complete reduction of surface oxides of green compact particles by emitted atomic hydrogen was carried out by further heating the green compact to 700° C. and holding at this temperature for 35 min when the green com...
example 3
[0107]A powder blend of three types of powders was used: (1) 70 wt. % of titanium hydride powder TiH2 containing 3.8 wt. % of hydrogen and having particle size less than 120 μm, (2) 20% wt. % of CP titanium powder, which does not contain hydrogen, particle size <150 microns, and (3) 10 wt. % of the 60Al-40V master alloy powder having particle size <65 μm.
[0108]These powders were mixed together, and the obtained mixed powder was compacted at 960 MPa to a low density green compact of 3.46 g / cm3.
[0109]The green compact having the thickness 16 mm was heated to 250° C. at a slow heating rate of ˜7° C. / min and held at this temperature for 50 min to release absorbed water from the powders. Then, heating was continued at a heating rate of ˜20° C. / min to 580-600° C. in the atmosphere of emitted atomic hydrogen and held at this temperature for 30 min to form β-phase titanium and release reaction water from the powder.
[0110]Almost complete reduction of surface oxides of green compact particles...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pressure | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com