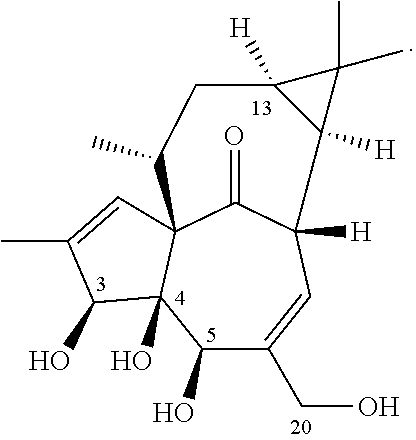
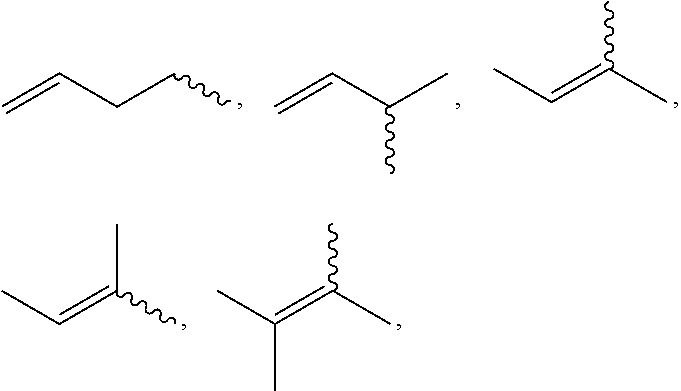
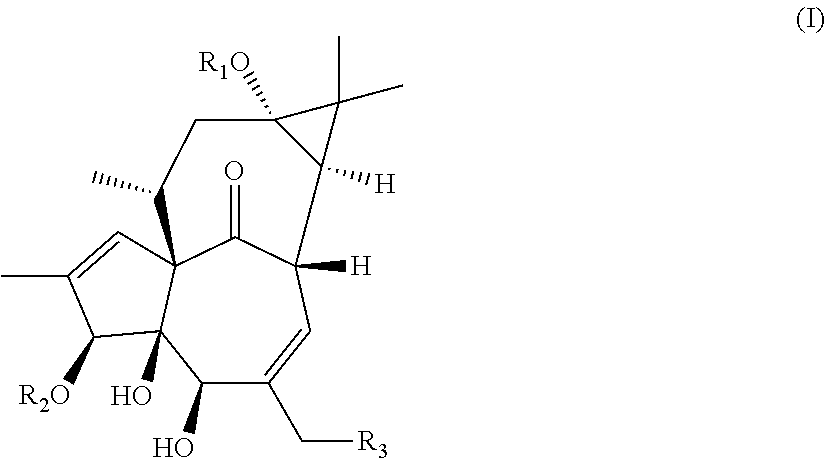
13-oxidized ingenol derivative and use thereof
a technology of ingenol and derivatives, applied in the field of derivatives of 13-oxidized ingenol, can solve problems such as limiting their us
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of 13-O-decanoyl-ingenol (Compound 1a), 13-O-(2′E,4′E-decadienoyl)-ingenol (Compound 1b) and 13-O-dodecanoyl-ingenol (Compound 1c), 20-O-deoxy-13-O-dodecanoyl-ingenol (Compound 1d), 6,7-epoxy-20-deoxy-ingenol (Compound 1e), 6,7-epoxy-20-O-(2,3-dimethylbutyryl)-13-O-dodecanoyl-ingenol (Compound 1f)
[0183]20 kg of kansui L. medicinal herbs was cold-soak extracted by 3 to 6 folds amount of 95% ethanol, until complete extraction; the extracting solutions were combined, and concentrated under reduced pressure until an alcohol-free taste to obtain an extract. To the extract, 10 folds amount of a solution of NaOH in methanol (0.5 M) was added, the mixture was stirred for about 2 hours. The mixture was adjusted to pH about 7 with dilute hydrochloric acid, and extracted 3 times with ethyl acetate, and then the ethyl acetate layers were combined, concentrated under reduced pressure to dryness. The extract was purified by normal phase silica gel column chromatography eluted with a g...
example 2
Preparation of 13-O-dodecanoyl-ingenol acetonide
[0198]13-O-dodecanoyl-ingenol (0.2 mmol) was dissolved in 0.5 mg / mL of a solution of p-toluenesulfonic acid monohydrate in acetone and stirred at 30° C. for 12 h. The reaction solution was concentrated to dryness under reduced pressure, re-dissolved in ethyl acetate, and washed with water and saturated aqueous sodium chloride solution, successively, and then the organic layer was concentrated to dryness under reduced pressure. The resulting residual was purified by flash chromatography (petroleum ether: ethyl acetate=10:1 to 6:4) to obtain 13-O-dodecanoyl-ingenol 3,4,5,20-diacetonide (Compound 2a), 13-O-dodecanoyl-ingenol 5,20-acetonide (Compound 2b), 13-O-dodecanoyl-ingenol 3,4-acetonide (Compound 2c), respectively.
[0199]The structural formula and hydrogen nuclear magnetic resonance spectrum (1H-NMR) of the compounds are as follows:
1) 13-O-dodecanoyl-ingenol 3,4,5,20-diacetonide (Compound 2a)
[0200]
[0201]1H-NMR (400 MHz, CDCl3) δ 5.93 ...
example 3
Preparation of 3-O-angeloyl-13-hydroxy-ingenol (Compound 3)
[0206]
[0207]13-O-dodecanoyl-ingenol 3,4,5,20-diacetonide (Compound 2a, for which the preparation procedure may refer to Example 2) (0.5 mmol) was dissolved in diethyl ether, and lithium aluminium hydride was added in the condition of ice water bath and stirred for 15 min. The reaction solution was added with a saturated aqueous sodium sulfate solution, and extracted with diethyl ether. The organic layer was washed with saturated aqueous sodium chloride solution, and then concentrated to dryness under reduced pressure. The resulting residual was purified by flash chromatography (petroleum ether: ethyl acetate=7:3) to obtain 13-OH-ingenol 3,4,5,20-diacetonide (Compound 31).
[0208]Compound 31 (0.4 mmol) was dissolved in methanol, 4 M diluted hydrochloric acid was added and stirred at room temperature for 15 h. The reaction solution was poured into water, adjusted to pH about 7 with sodium hydrogen carbonate solution, and extract...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Density | aaaaa | aaaaa |
| Density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com