Genetic assay for protein nuclear transport
a gene assay and protein technology, applied in the field of gene assays, can solve the problems of not representing a functional signal, difficult to predict the presence of proteins in a given protein, and both methods have serious technical disadvantages, and achieve the effect of decreasing the expression level of reporter genes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example i
Construction of Vectors for the Genetic Assay of Nuclear Import
[0085]These constructs are designed to express fusion proteins composed of three functional parts: a modified LexA protein, an activation domain of the GAL4 protein, and a protein to be tested for its nuclear import. These components were obtained and joined together as follows:
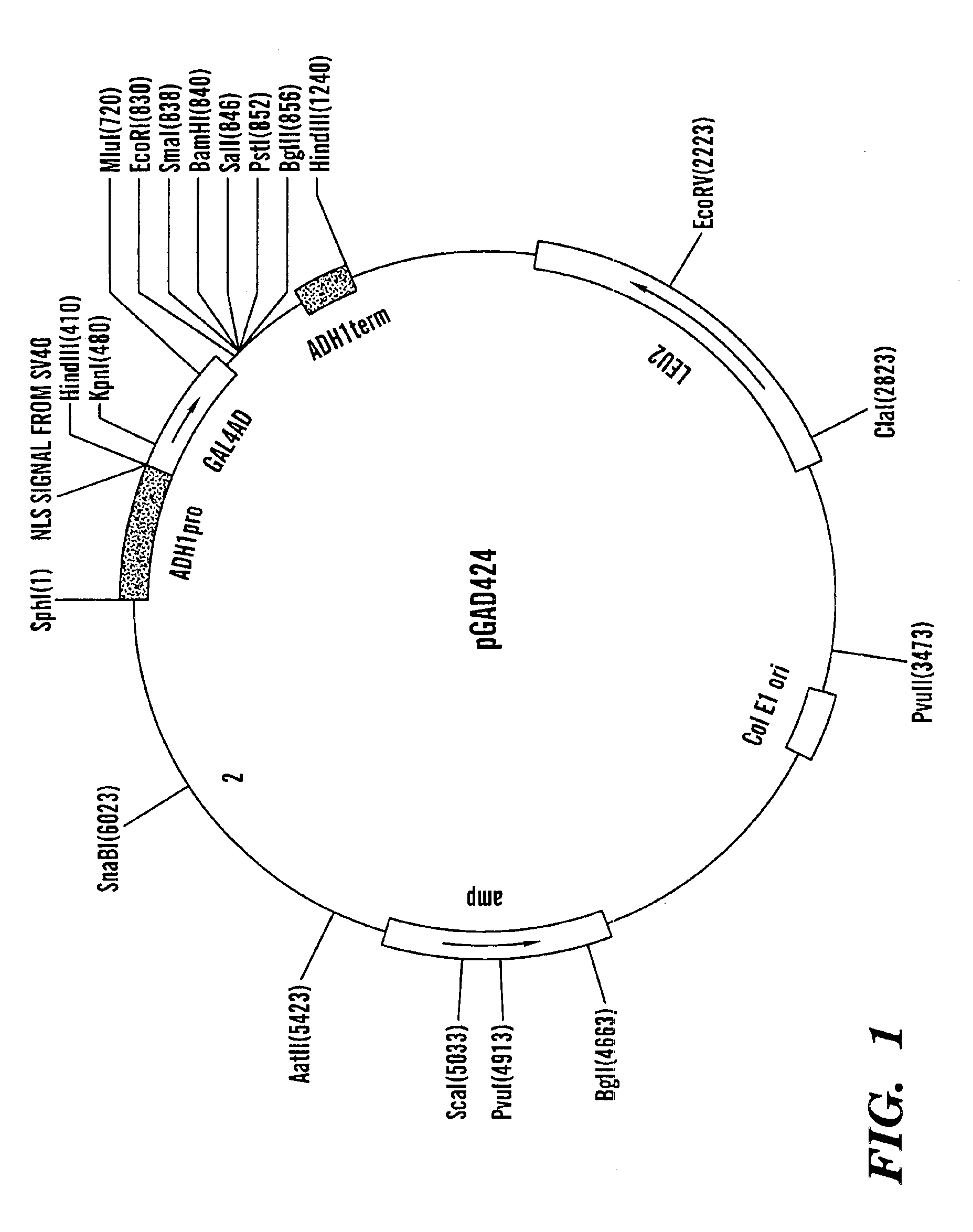
[0086](A) First, the Gal4 activation domain (AD), derived from the pGAD424 plasmid (FIG. 1), was PCR-amplified with and without the adjacent SV40 NLS (AD with NLS was used for positive control constructs, see below). During amplification, EcoRI and BamHI restriction sites were introduced at the 5′ and 3′ ends of the amplified fragment, respectively. The PCR mixtures contained the following components:
[0087]
(a) PCR of GAL4 AD without NLSPrimer GAD5 (20 μM)5μlPrimer GAD3BdE (20 μM)5μldNTPs (10 mM each for dATP,2μldTTP, dGTP, dCTP)Pfu reaction buffer (10X)10μlTemplate DNA (pGAD424, 10 ng / μl)5μlPfu polymerase (0.5μ / μl)1μlDouble distilled water72μlTOTA...
example ii
One-Hybrid Genetic Assay for Protein Nuclear Import
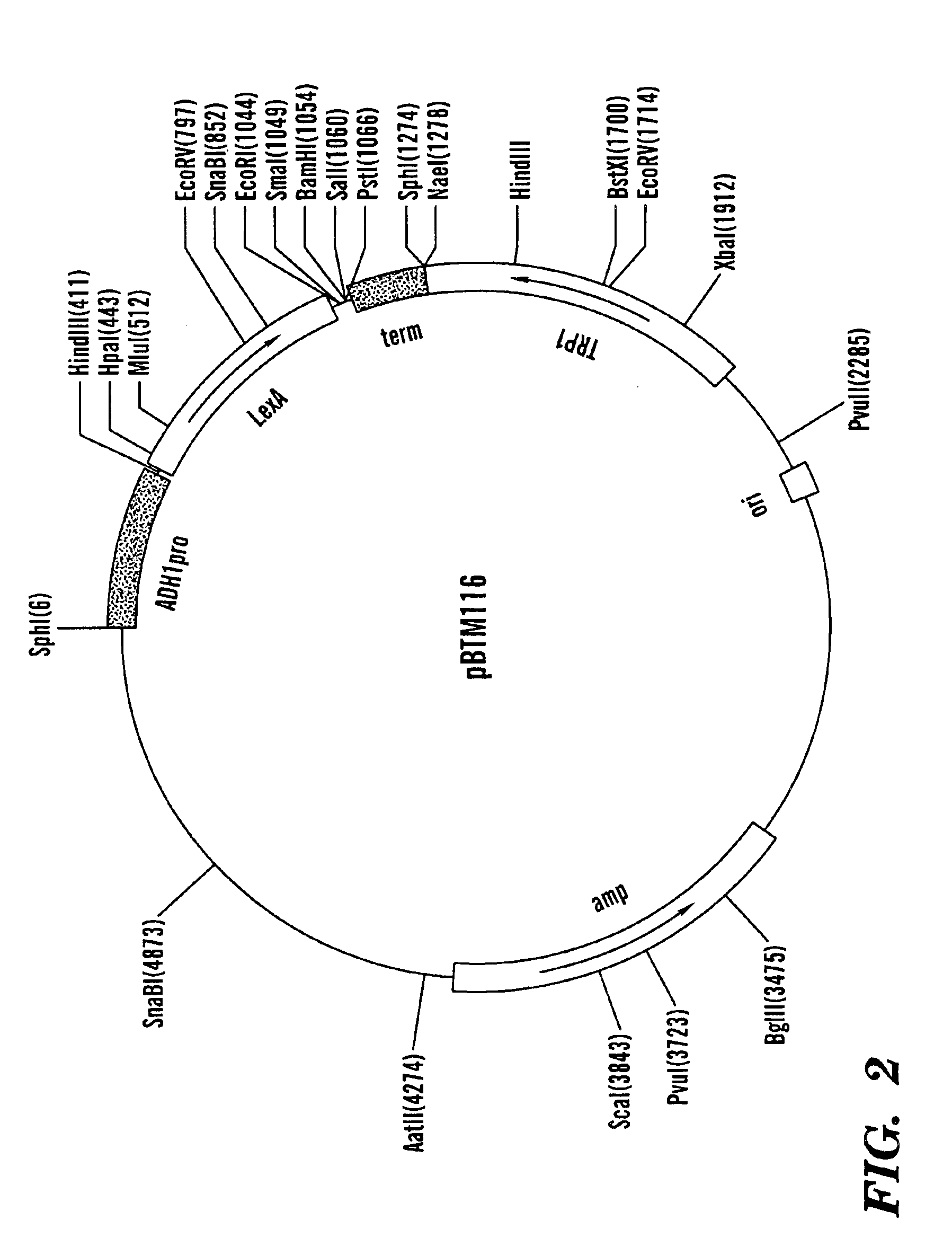
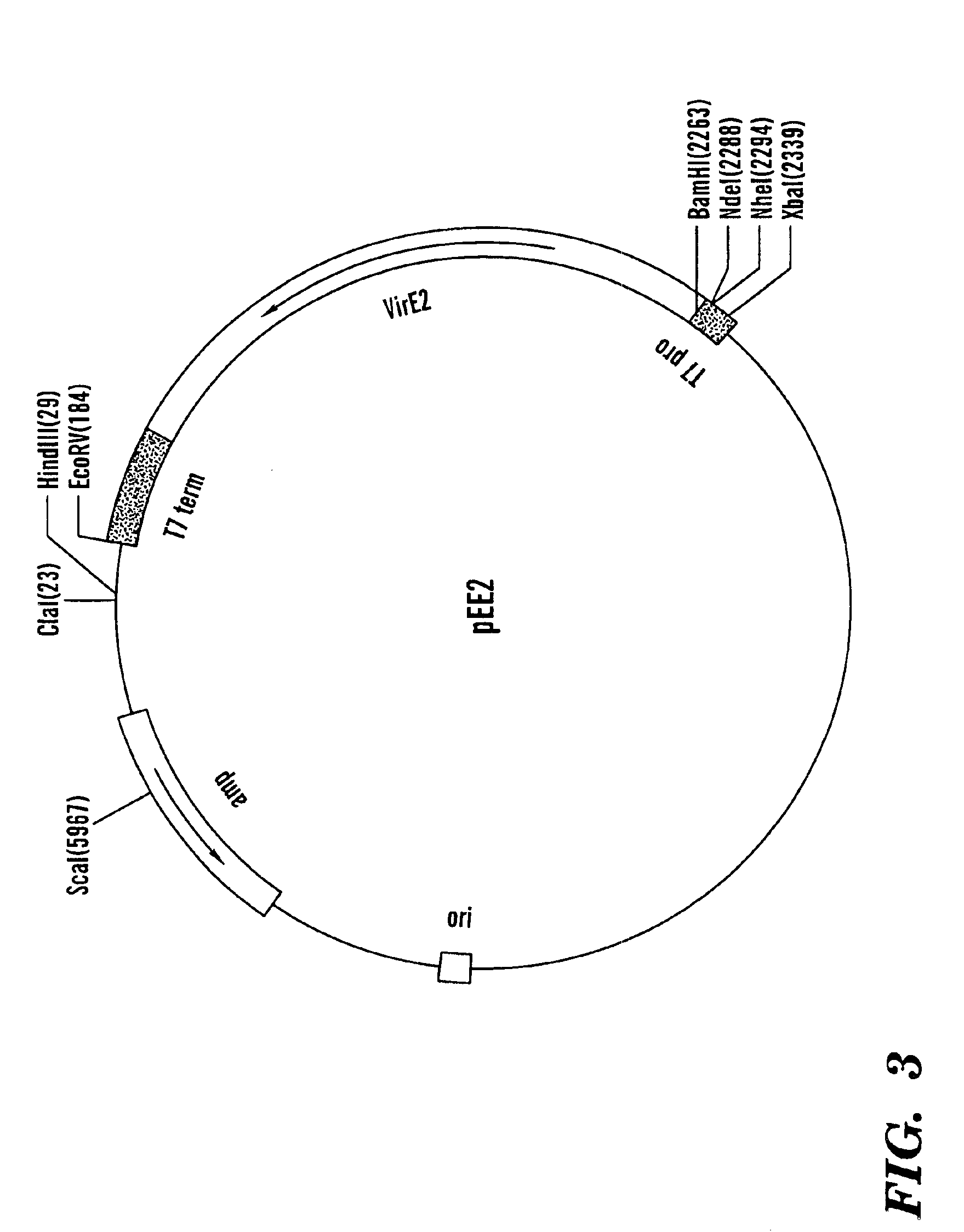
[0103]The fusion protein derived from pLexA::GAL4AD (−)NLS::VirE2 enters the cell nucleus and activates the reporter gene expression, indicating that LexA carries a cryptic NLS although it is a prokaryotic protein and is not expected to enter the nucleus. Thus it was necessary to identify and disable this signal, resulting in a modification of the LexA protein (see item D above).
[0104]The fusion protein derived from pmLexA:: GAL4AD (−)NLS, which lacks a tested protein, enters the cell nucleus by diffusion due to its small size (approximately 38 kDa). This import, however, is less efficient than that of NLS-containing fusion products, resulting in a weaker expression of the β-galactosidase reporter. Nevertheless, it is recommended to use pmLexA::GAL4AD (−)NLS::VirE2 as a negative control for the assay. This 106 kDa fusion protein does not enter the nucleus at all, producing zero expression of the reporter.
[0105]The user has an option...
example iii
[0109]Nuclear import assay. The basic strategy of these experiments is based on expression in yeast cells of a triple-fusion protein comprising bacterial LexA, yeast Gal4 activation domain (Gal4AD), and the tested protein encoded by a cDNA subcloned in-frame downstream of Gal4AD (FIG. 16). If the tested protein contains a functional NLS, the fusion product will enter the yeast cell nucleus. Following this nuclear import, the LexA domain will target the fusion protein to the LexA operator sites of the reporter lacZ gene contained in the L40 yeast strain. Gal4AD then activates the expression of lacZ, resulting in β-galactosidase activity. In the absence of a NLS, the fusion protein is unable to reach the cell nucleus and, thus, activate the reporter gene.
[0110]In addition to induction of the β-galactosidase reporter, this one-hybrid system allows direct selection for the nuclear import of the tested protein in the same L40 yeast strain, which contains an integrated copy of the HIS3 ge...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Transport properties | aaaaa | aaaaa |
| Level | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com