C-nitroso compounds and use thereof
a technology of c-nitroso compounds and compounds, which is applied in the field of c-nitroso compounds, can solve the problems that c-nitroso compounds derived from carbon acids with lower acidities (higher pka values) will not act as useful donors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example i
Synthesis of Dimeric 2-[4′-(α-Nitroso)isobutyrylphenyl]propionic Acid
[0237]The synthesis of dimeric 2-[4′-(α-nitroso)isobutyrylphenyl]propionic acid was carried out according to the reaction scheme for this set forth above as follows:
[0238]To a solution of ibuprofen 1A (9.89g, 48 mmol) in anhydrous EtOH (35 mL) was added chlorotrimethylsilane (18.27 mL, 144 mmol) at room temperature, and the mixture was stirred at the same temperature for 2 h. After the removal of the excess EtOH and chlorotrimethylsilane under reduced pressure, the oily residue was treated with ice-cold saturated NaHCO3 (150 mL), and the resulting mixture was extracted with hexanes (450 mL). The hexanes solution was washed with brine (3×50 mL), and dried over anhydrous Na2SO2. Evaporation of the solvent afforded ethyl 2-(4′-isobutylphenyl)propionate 2A (11.24 g, in 100% yield) as a colorless oil.
[0239]Ester 2A (11.23 g, 48 mmol) was added dropwise to a stirred suspension of CrO3 (20.8 g, 208 mmol) in acetic acid (A...
example ii
[0243]The ability of various C-nitroso compounds as described below to relax a rabbit aortic ring (smooth muscle) was carried out as described in Stamler, J., et al., PNAS, Vol. 89, 444-448 (1992).
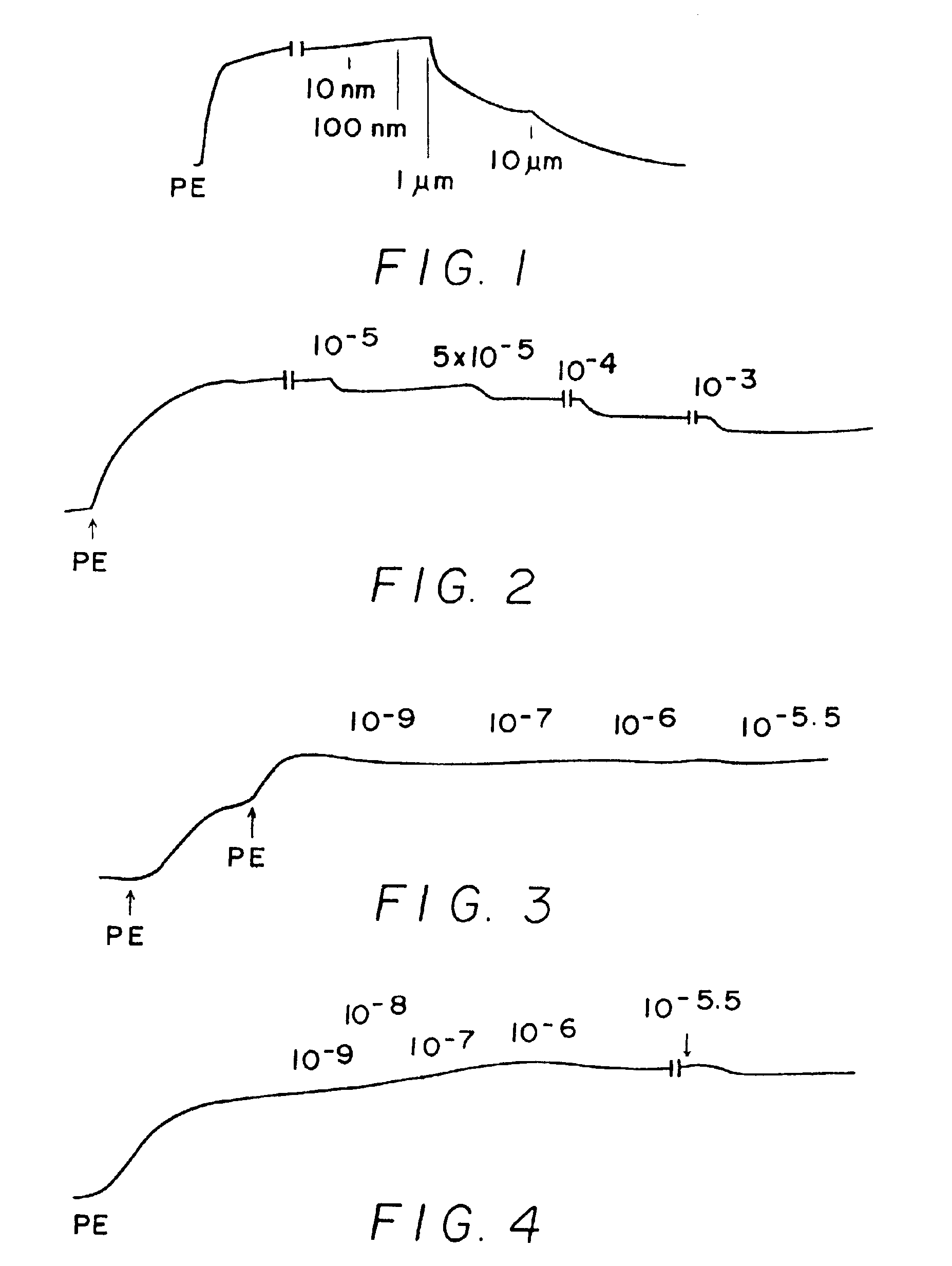
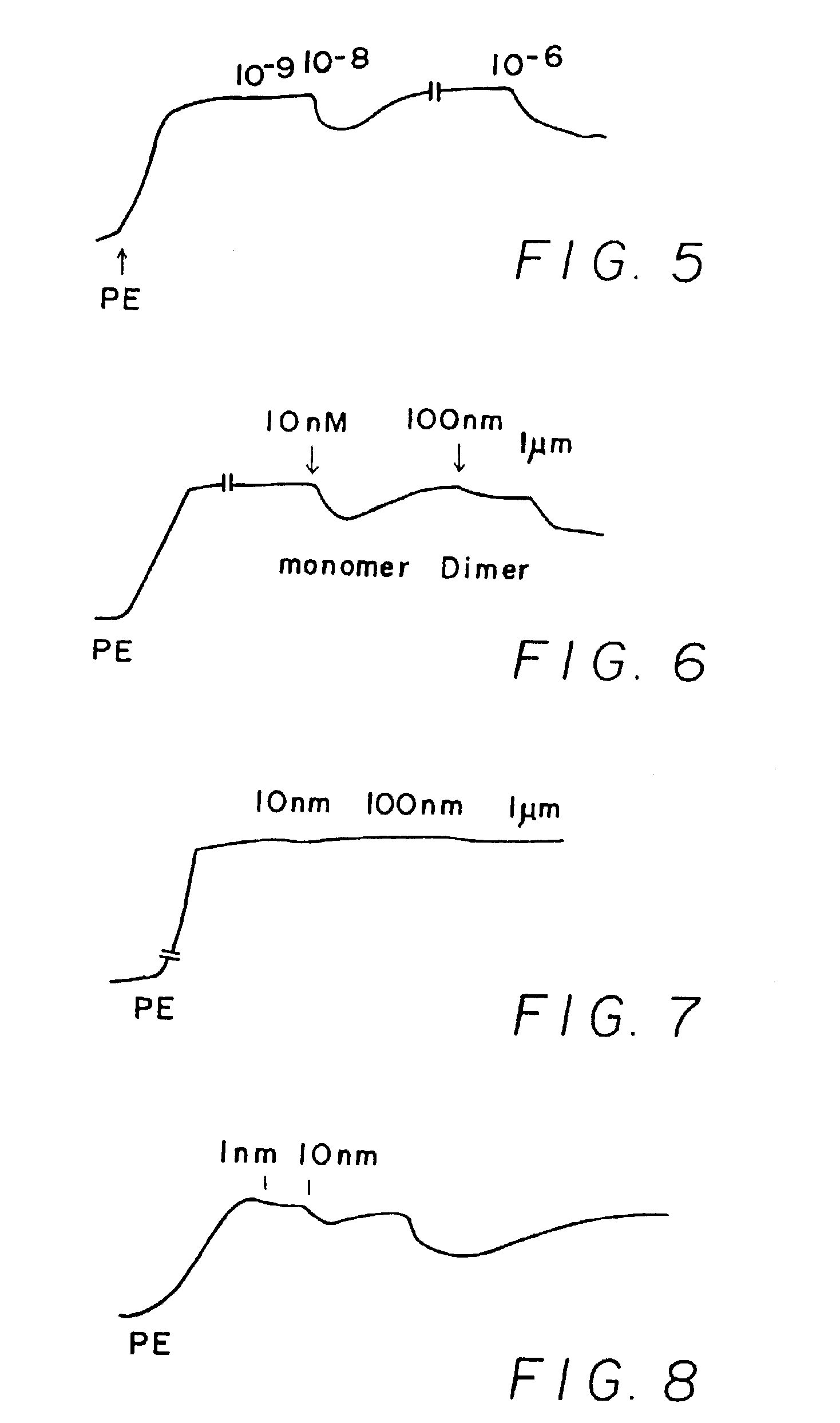
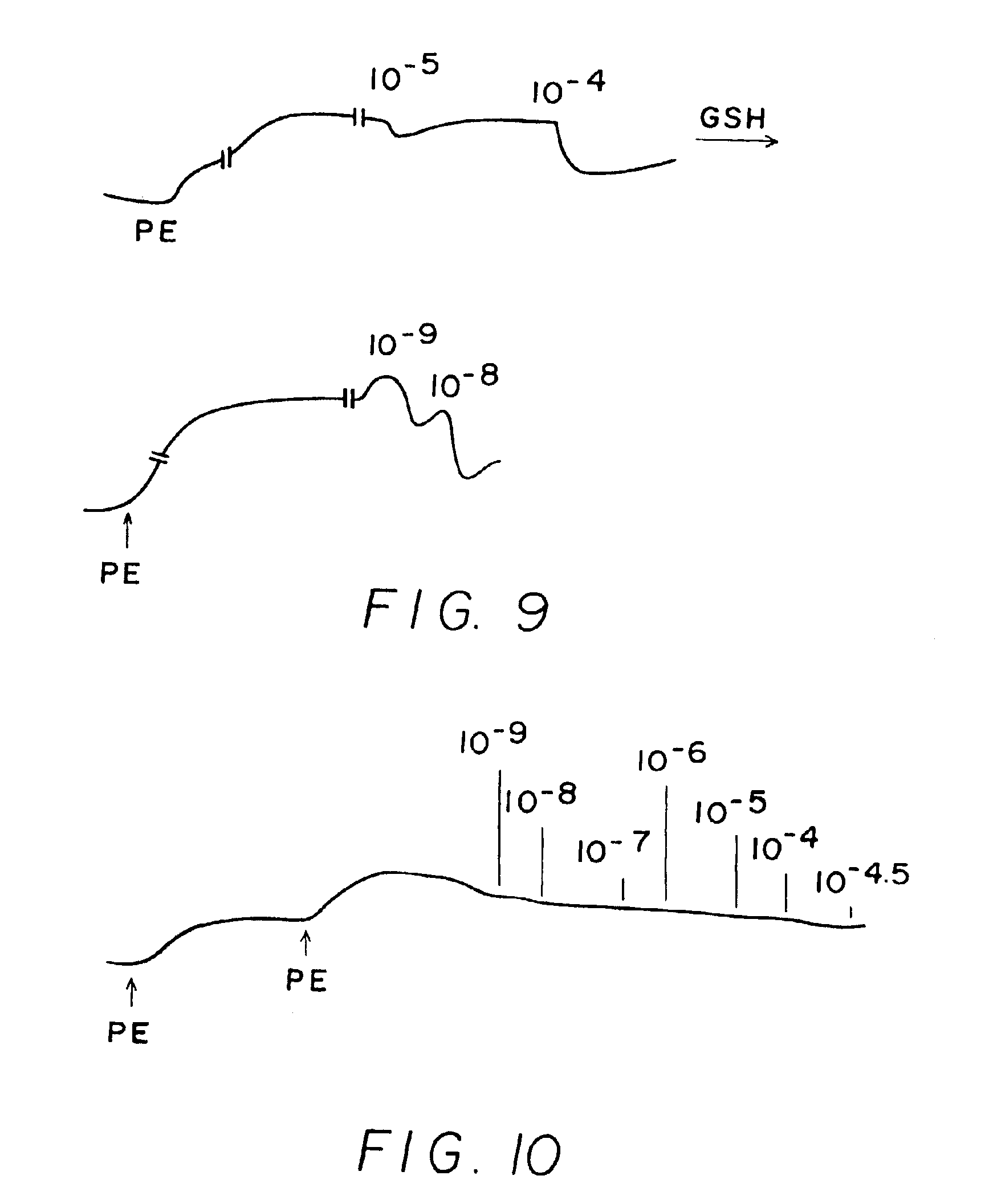
[0244]Results shown in FIGS. 1-10 which are tracings of force (tension) in the Y-direction versus time in the X-direction with downward direction indicating relaxation and upward direction indicating constriction. Concentrations of C-nitroso compound applied at time in the X-direction are indicated as 10−9 (1 nanomolar), 10−6 (1 micromolar), 10−3 (1 milimolar), etc. “PE” on the figures means the application of phenylephrine, a constricting agent.
[0245]FIG. 1 shows results for Compound (129a) which is a C-nitroso compound obtained by nitrosylating a carbon acid with a pH less than 10. Relaxation effect is shown in FIG. 1 at 10 μM concentration, i.e., at micromolar concentrations.
[0246]FIG. 2 shows results for C-nitroso-methylmalonic acid. It is obtained from a carbon acid with a pKa of abou...
example iii
[0255]A 60-year-old white male with arthritis, esophageal spasm, coronary artery disease, congestive heart failure, impotence and nightly urinary incontinence develops gastrointestinal upset when administered ibuprofen (400 mg, three times a day). When the drug is changed so that 0.1% by weight of the drug is administered as the dimeric nitrosoketoibuprofen of Example I, all symptoms are relieved.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pKa | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com