Monomeric streptavidin muteins
a technology of monomerization and streptavidin, which is applied in the field of monomerization of streptavidin, can solve the problems of difficult complete denaturation, high cost of denaturation and renaturation process, and limited efficiency of purification and immobilization, and achieve the effect of further enhancing the efficiency of streptavidin monomerization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Construction of Streptavidin Mutants
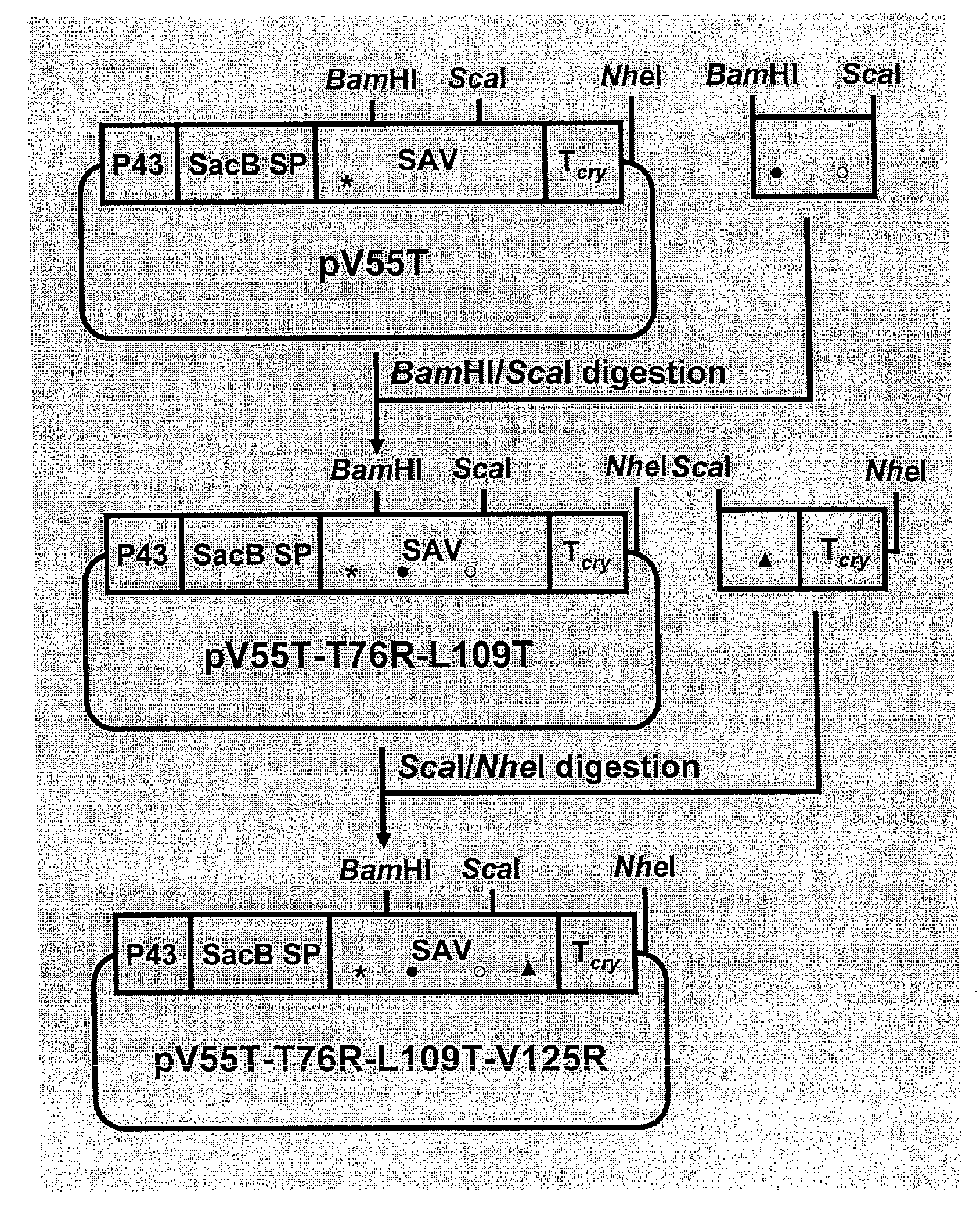
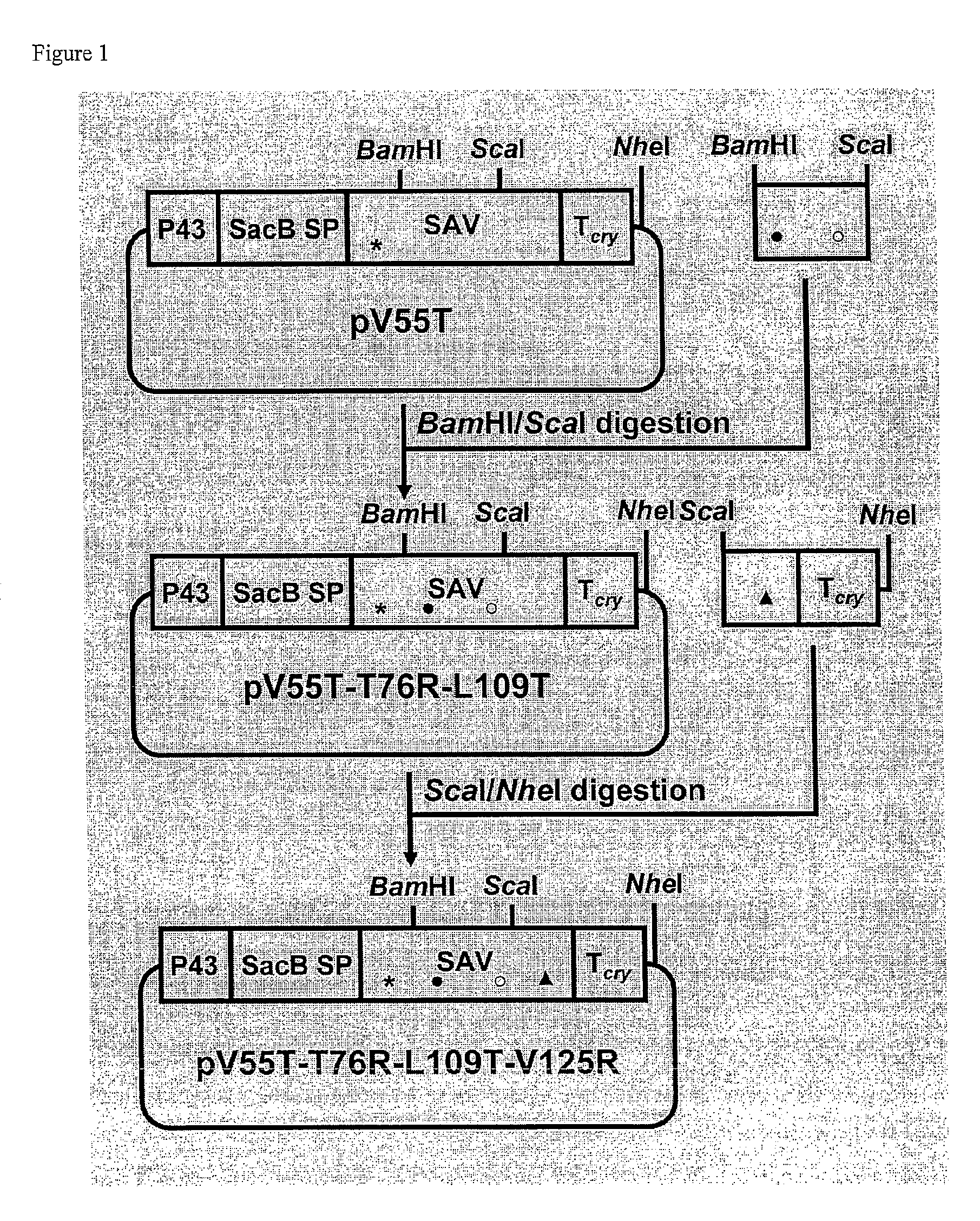
[0075]Different point mutations were introduced to the coding sequence of a synthetic streptavidin gene (ssav) (SEQ ID NO:3) in the B. subtilis expression vector pSSAV-T cry (Wu, 2002) by PCR-based oligonucleotide-directed mutagenesis. Five mutants (V125R, V125T, V55R, V55T and T76R), each bearing a single mutation which results in the change of an amino acid residue as the name suggests, were constructed using pSSAV-T cry as the template and the primers listed in Table 3. The amplified products were digested with the pair of enzymes listed in Table 3 and cloned into pSSAV-T cry. Five plasmids (pV125R, pV125T, pV55 R, pV55T and pT76R) resulted.
[0076]Two double mutants (M2 and prior art AK) were also constructed. For M2 (T76R, V125R), a ScaI / NheI digested fragment of pV125R was used to replace the corresponding fragment in pT76R. For AK (D61A, W120K), the fragment bearing the two mutations was amplified by PCR using the primers SAVD61AF and SAVW120...
example 2
Production and Purification of Streptavidin
[0079]Wild-type streptavidin (SEQ ID NO. 13) was produced by B. subtilis WB800 [pSSAV-T cry] cultured in a defined medium (Wu, 2002). The secreted protein was purified to homogeneity using cation exchange followed by iminobiotin affinity chromatography (Qureshi, 200). Production and purification of streptavidin muteins followed a similar scheme with two major modifications: super-rich medium (Halling, 1977) was used in place of the defined medium and biotin-agarose (Sigma) was used in place of iminobiotin agarose as the affinity matrix. Dialyzed sample containing partially purified mutein was loaded to a 1-ml biotin-agarose column. Streptavidin muteins were eluted from the column using 20 mM d-biotin in phosphate-buffered saline (PBS; 50 mM sodium phosphate, 100 mM NaCl, pH 7.2). Concentration of purified streptavidin was determined spectrophotometrically using the known extinction coefficient at 280 mm (Gill, 1989; Gasteiger, 2003) for eac...
example 3
Determination of the Molecular Size of Streptavidin
[0080]Molecular mass of purified streptavidin and its muteins was estimated by both gel filtration and dynamic light scattering studies. Gel filtration was performed on the Bio-Rad biologic workstation equipped with a Bio-Prep SE 100 / 17 column that had been calibrated with molecular mass protein markers (Bio-Rad). Molecular mass was also estimated from the hydrodynamic radius of the mutein obtained using a DynaPro MS dynamic light scattering instrument (Protein Solutions, USA) that had been calibrated with lysozyme. Protein samples (2-3 mg / ml in PBS) were passed through a 0.02 μm filter (Whatman Anodisc 13) immediately prior to measurement. The size distribution profile was analyzed using the manufacturer's Dynamics V6 software.
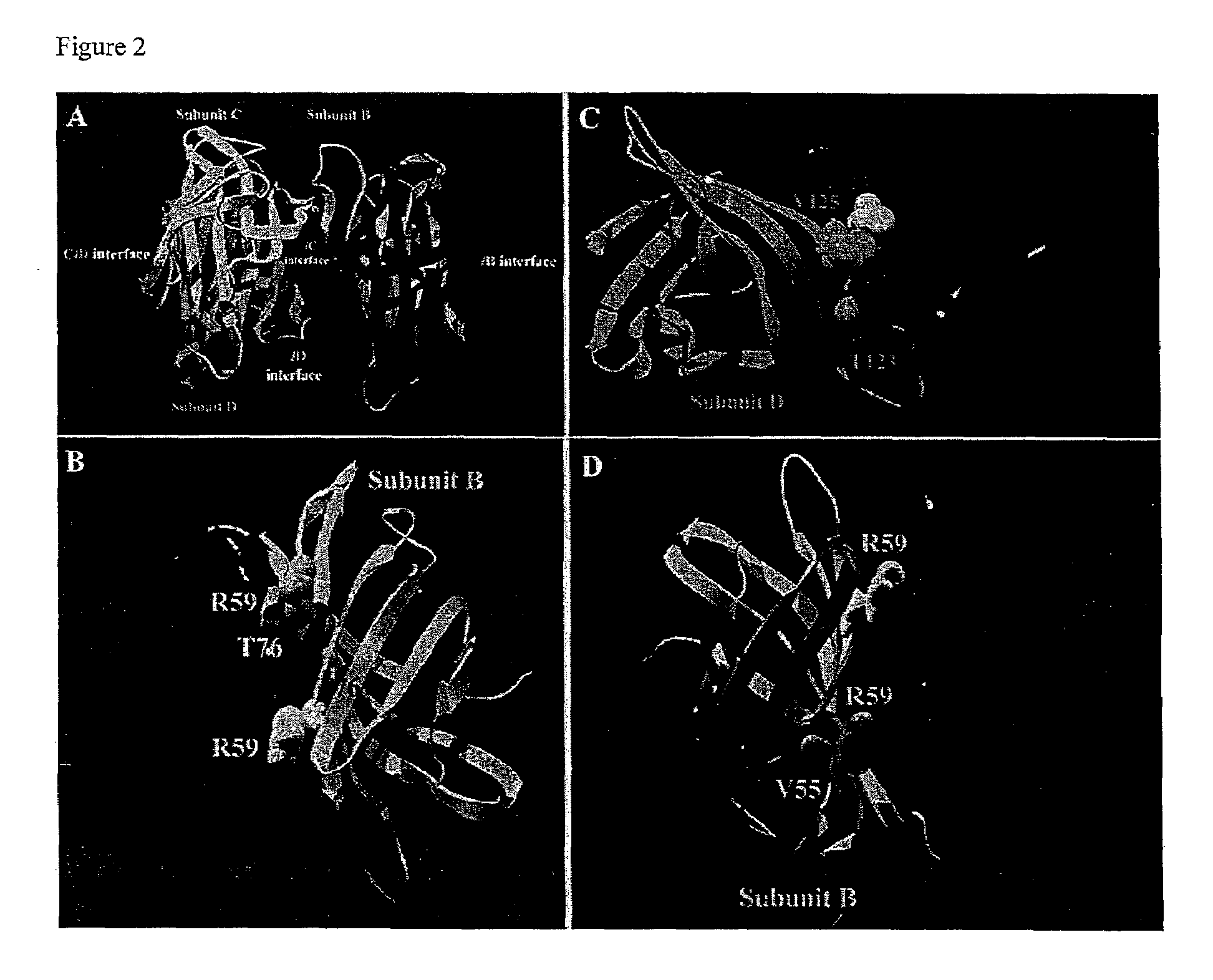
[0081]In single mutation muteins, the impact of the mutation on weakening of the subunit interaction followed the order T76R>V125R>V125≈V55R>V55T (FIG. 3 and Table 4). The T76R mutein (referred to herein as M...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com