Nickel Hydroxide electrode material with improved microstructure and method for making the same
a technology of nickel hydroxide and electrode material, which is applied in the field of positive electrode material to achieve the effect of improving capacity, rate capability, utilization and/or high temperature performan
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
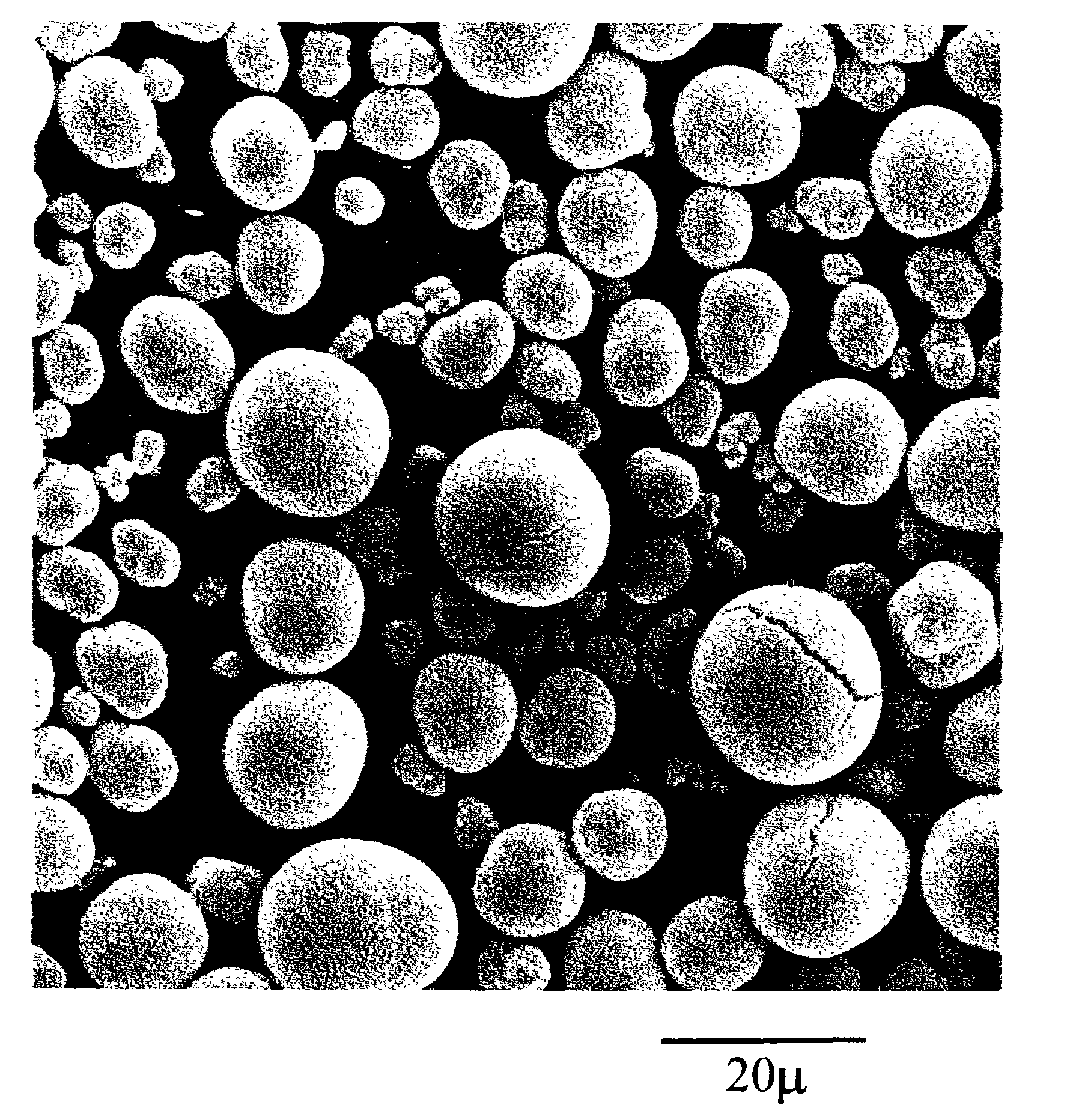
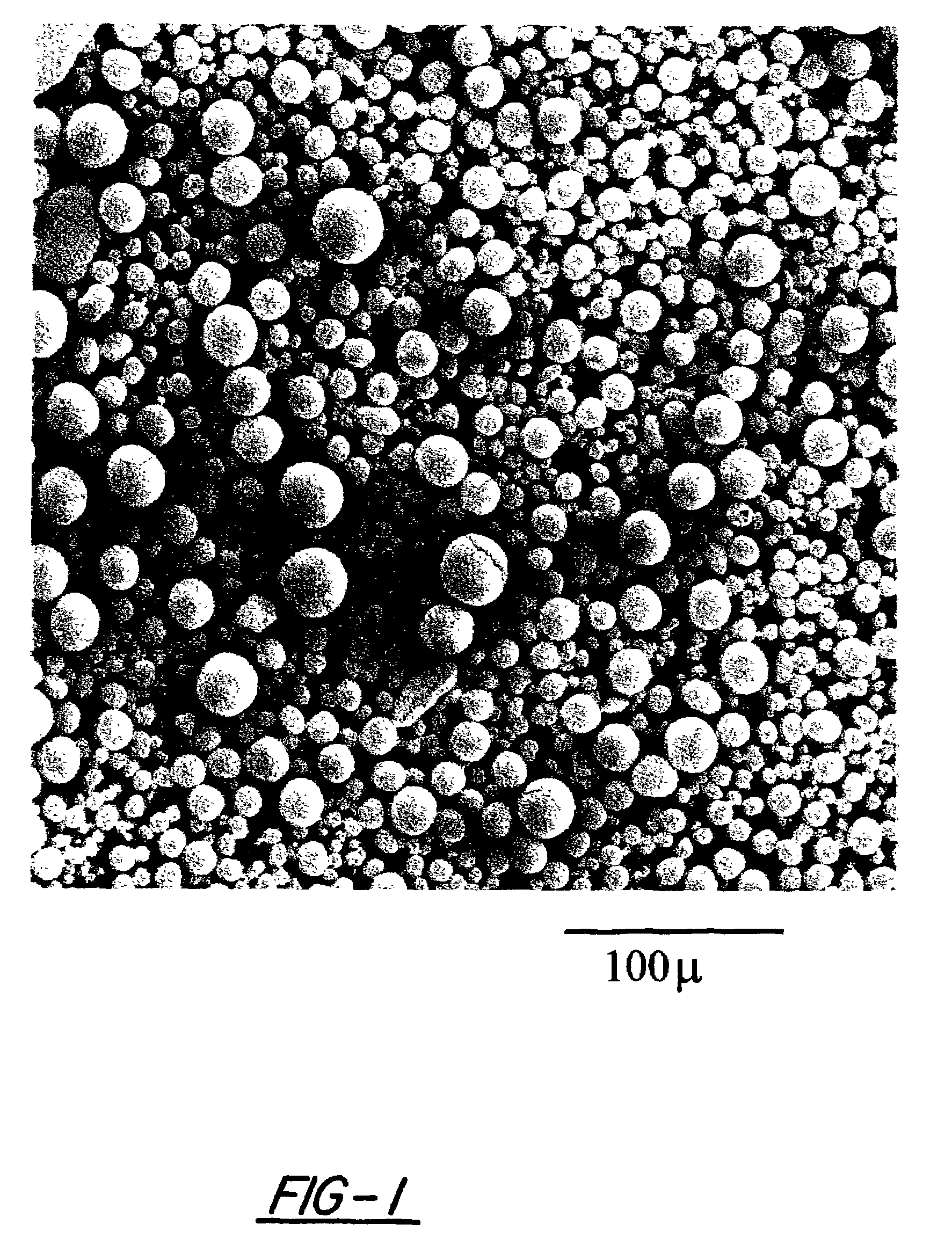
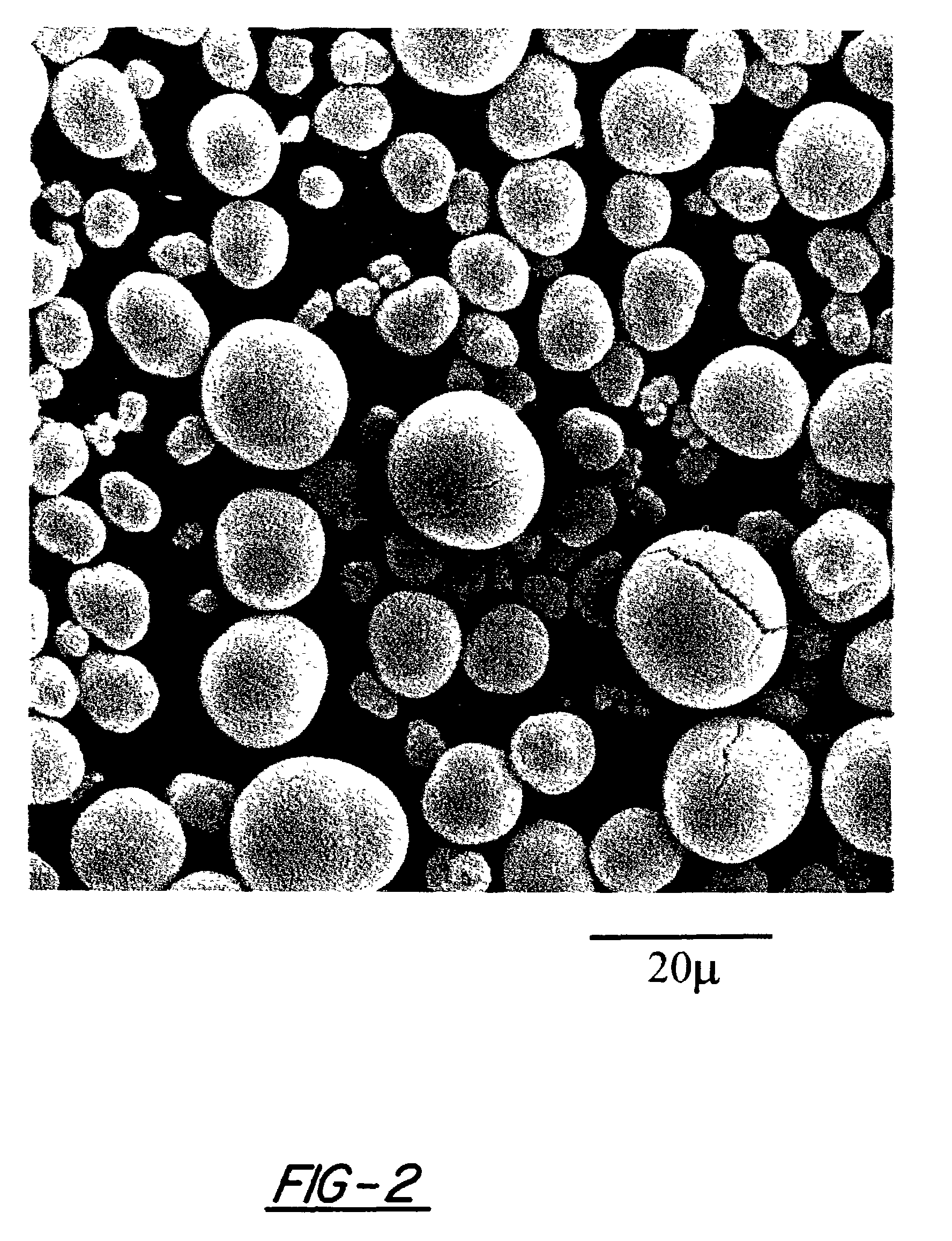
[0124]Slurries of the three formulations of nickel hydroxide material set forth in Table 1 above were formulated in a conventional manner. Such slurry was pasted onto expanded nickel foam, and dried to form positive electrodes. Loading for each of these electrodes is presented in Table 2. The electrodes formed from the modified nickel hydroxide materials of the instant invention were found to have much higher loading factors compared to conventional material. The higher loading of the modified nickel hydroxide materials of the present invention is the result of the uniformly spherical condition of the nickel hydroxide particles and their uniform size distribution (provides for a higher packing density).
[0125]Negative electrodes were fabricated as described in commonly assigned U.S. Pat. No. 5,536,591 (the disclosure of which is specifically incorporated herein by reference).
[0126]The negative metal hydride and the positive nickel hydroxide electrodes were used in flooded half cells ...
example 3
[0129]C type cells as prepared in Example 2 above, were subjected to capacity testing at different temperatures. The results are shown in FIG. 4.
PUM
| Property | Measurement | Unit |
|---|---|---|
| tap density | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| peak power | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com