MRI contrast agents
a contrast agent and mri technology, applied in the field of in vivo reagents, can solve the problems of inapplicability of antibody-based contrast agents, almost no mri contrast agents developed for such purposes, etc., and achieve the effect of easy preparation and outstanding utility in early detection and early treatmen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of the Contrast Agent
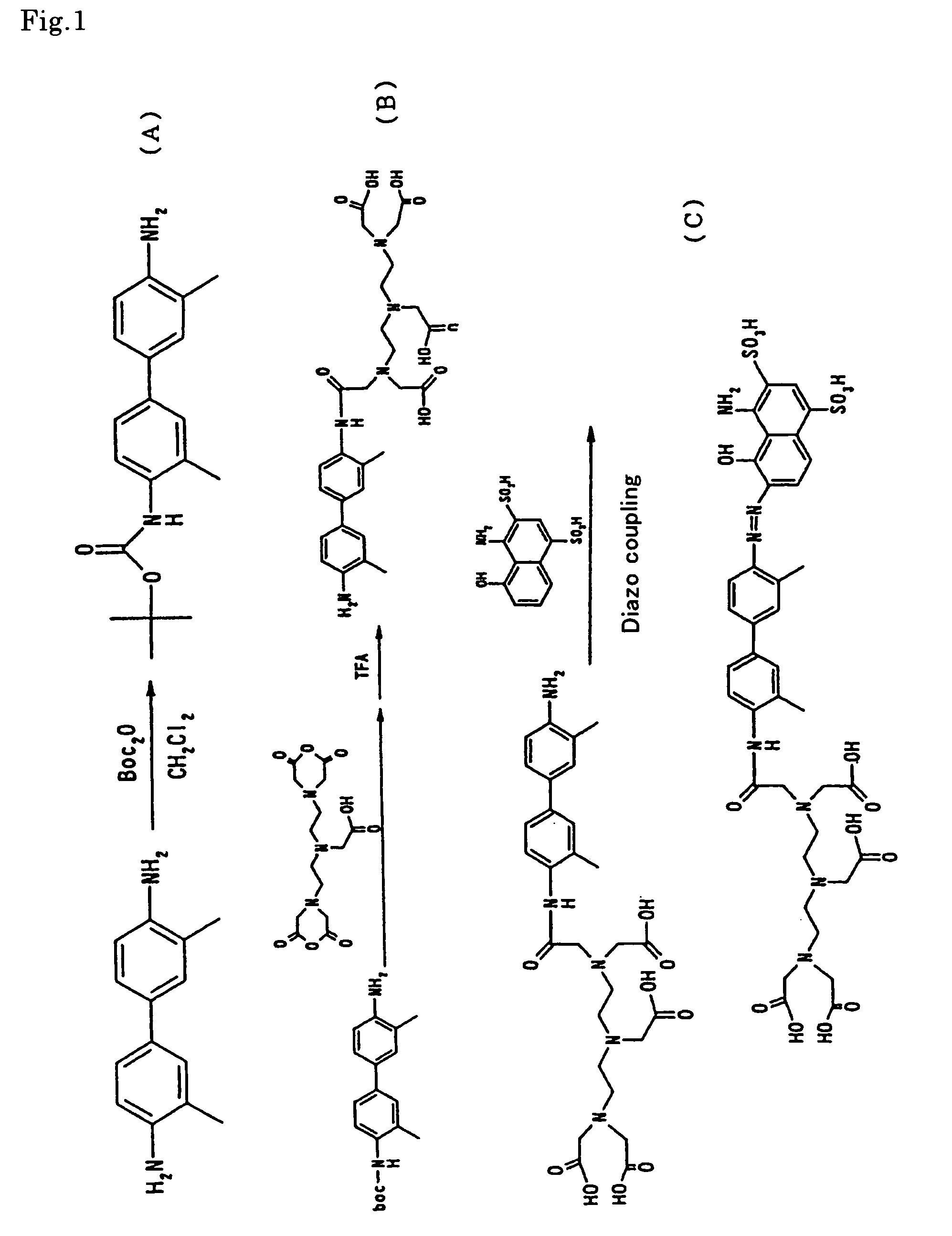
[0031]In accordance with the reaction scheme as shown in FIG. 1, there was synthesized the MRI contrast agent of the present invention as expressed by the aforementioned formula (VII).
(1) Synthesis of Boc DMB (Step A).
[0032]Dimethylbenzidine (DMB) 1.0 g (4.71 mmol), dibutoxycarbonylketone (Boc2O) 1.28 g (5.88 mmol) and triethylamine 0.714 g were added to 20 ml of dichloromethane, followed by stirring at room temperature for 48 hours. The reaction mixture was subjected to filtration, added with chloroform, and then washed with a saturated aqueous solution of L-sodium tartrate to remove unreacted DMB. The chloroform phase was subjected to concentration in vacuo, refining by silica gel chromatography, and then vacuum drying to obtain the desired product (0.60 g).
(2) Synthesis of DMB-DTPA (Step B).
[0033]Boc DMB 0.30 g (0.96 mmol) was dissolved in 20 ml of pyridine. In the resultant solution was suspended DTPA anhydride 0.343 g (0.96 mmol). The atmosphere o...
example 2
Evaluation of Imaging Capability by MRI (1)
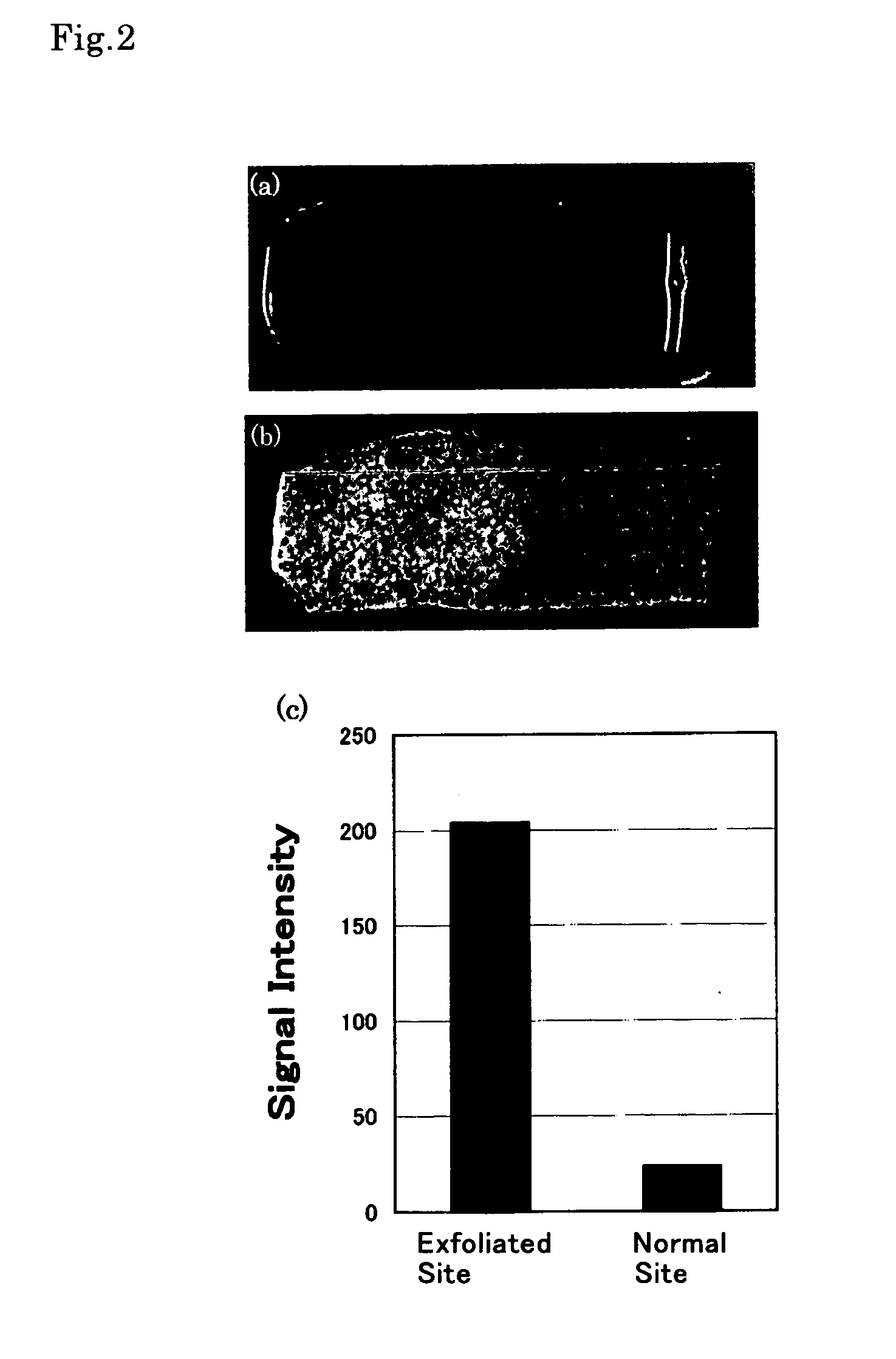
[0036]For the contrast agent as synthesized (prepared) in Example 1, evaluation of in vitro imaging capability by MRI was carried out with an aortic vascular section extirpated from a pig, as a specimen.
[0037]The aortic vascular section extirpated from a pig was spread in a rectangular shape measuring about 2 cm×3 cm to prepare a vascular section sample. The endothelial surface of the left half of the sample was exfoliated with a scalpel. The sample was then immersed in a 10 mM aqueous solution of the contrast agent for ten seconds. Following thorough washing with physiological saline solution, the sample was subjected to the evaluation by MRI as well as by visual observation.
[0038]The results are shown in FIG. 2. FIG. 2 (A) is a picture taken with a digital camera, which corresponds to the visual observation. It was visually observed that the exfoliated endothelial site (the left half) was colored blue, suggesting accumulation of the contr...
example 3
Evaluation of Imaging Capability by MRI (2)
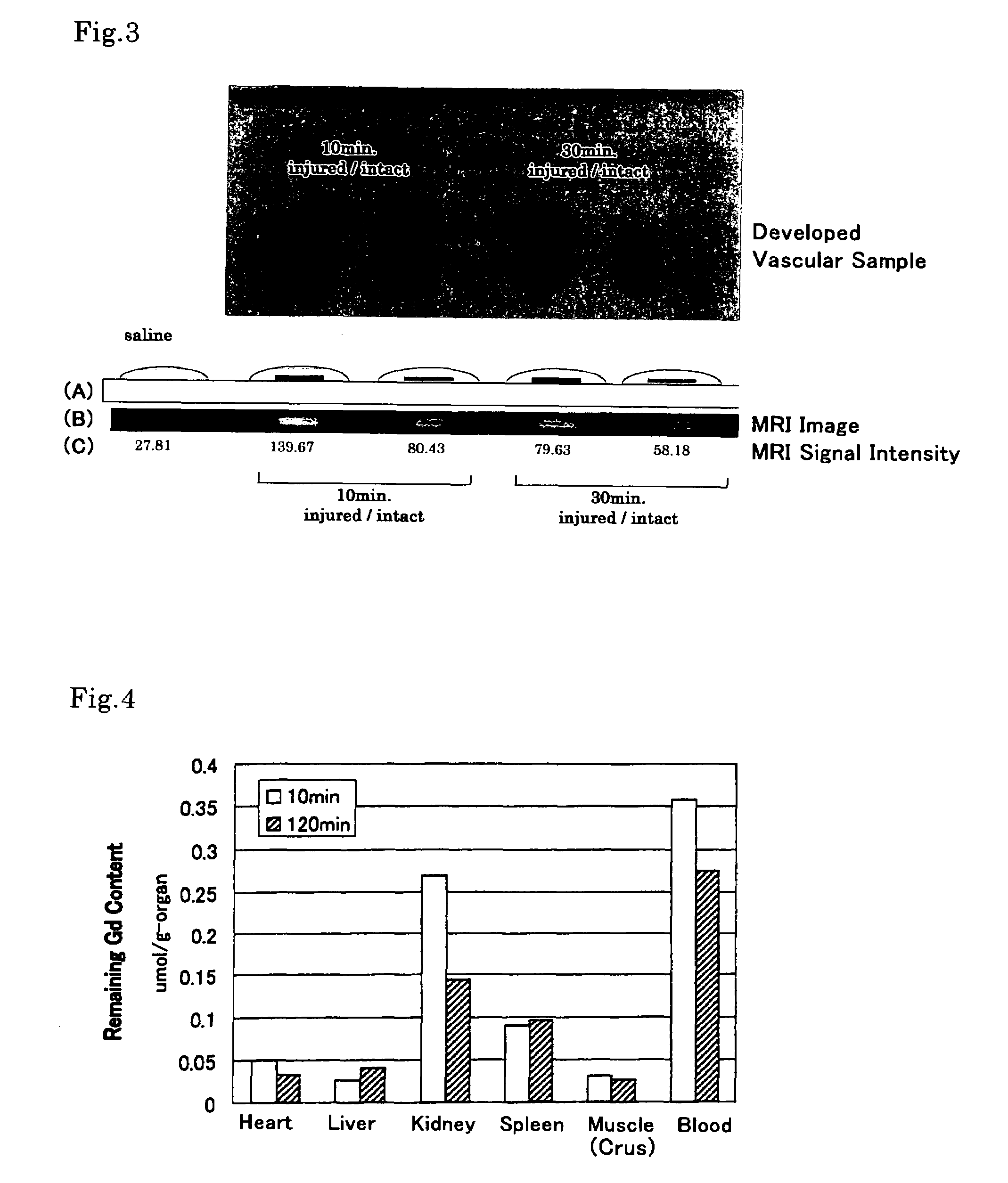
[0039]The contrast agent of the present invention was evaluated for imaging capability by means of an ex vivo experiment using a living rat.
[0040]A balloon-tip catheter was passed through the left femoral artery of the rat to injure the carotid artery with the balloon. Then 2 ml of aqueous saline solution containing 24 mM of the contrast agent (EBDTPA-Gd prepared in Example 1) was injected to the rat through the right jugular vein. After a predetermined period of time (10, 30 or 120 minutes), the right carotid artery and the left carotid artery were extirpated, developed, washed with a physiological saline, and then subjected to evaluation by MRI. The MRI evaluation was carried out by imaging the extirpated carotid artery sections, under the condition of being added with one or two droplets of physiological saline solution, by the T1 weighted spin echo method.
[0041]The results are shown in FIG. 3. In FIG. 3, by “injured” is denoted the exfo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com