Preparation of metal mesoporphyrin compounds
a technology of mesoporphyrin and halide compounds, which is applied in the direction of organic chemistry, iron group organic compounds without c-metal linkages, iron organic compounds, etc., can solve the problems of difficult to drive the reaction to completion, degradation of intermediate products, and process unsuitable for industrial scale up, so as to reduce degradation and increase degradation. , the effect of short reaction tim
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0033]Preparation of Mesoporphyrin IX Formate
[0034]A 2000 ml hydrogenation vessel was charged with 40.0 g hemin, 4.0 g 5% Pd / C (50% water by weight), and 800 ml 96% formic acid. Since hemin and mesoporphyrin IX formate as well as all reaction intermediates are reportedly light sensitive materials, care was taken throughout this entire procedure to minimize the exposure of the reaction to visible or ultraviolet light.
[0035]The vessel was flushed with a nitrogen flow for 10 minutes. With vigorous stirring, it was then pressurized to 50 psi with hydrogen for ten minutes; then depressurized, and the cycle repeated. The vessel was further pressurized to 50 psi with hydrogen and the temperature was raised to 90° C. over approximately 20 minutes.
[0036]The hydrogenation reaction was maintained at 90° C. and 45-55 psi for 1-1.5 hours. The reaction mixture was not stable for extended periods of time at 90° C. The time at this temperature was sufficient to dissolve all hemin and convert the ma...
example 2
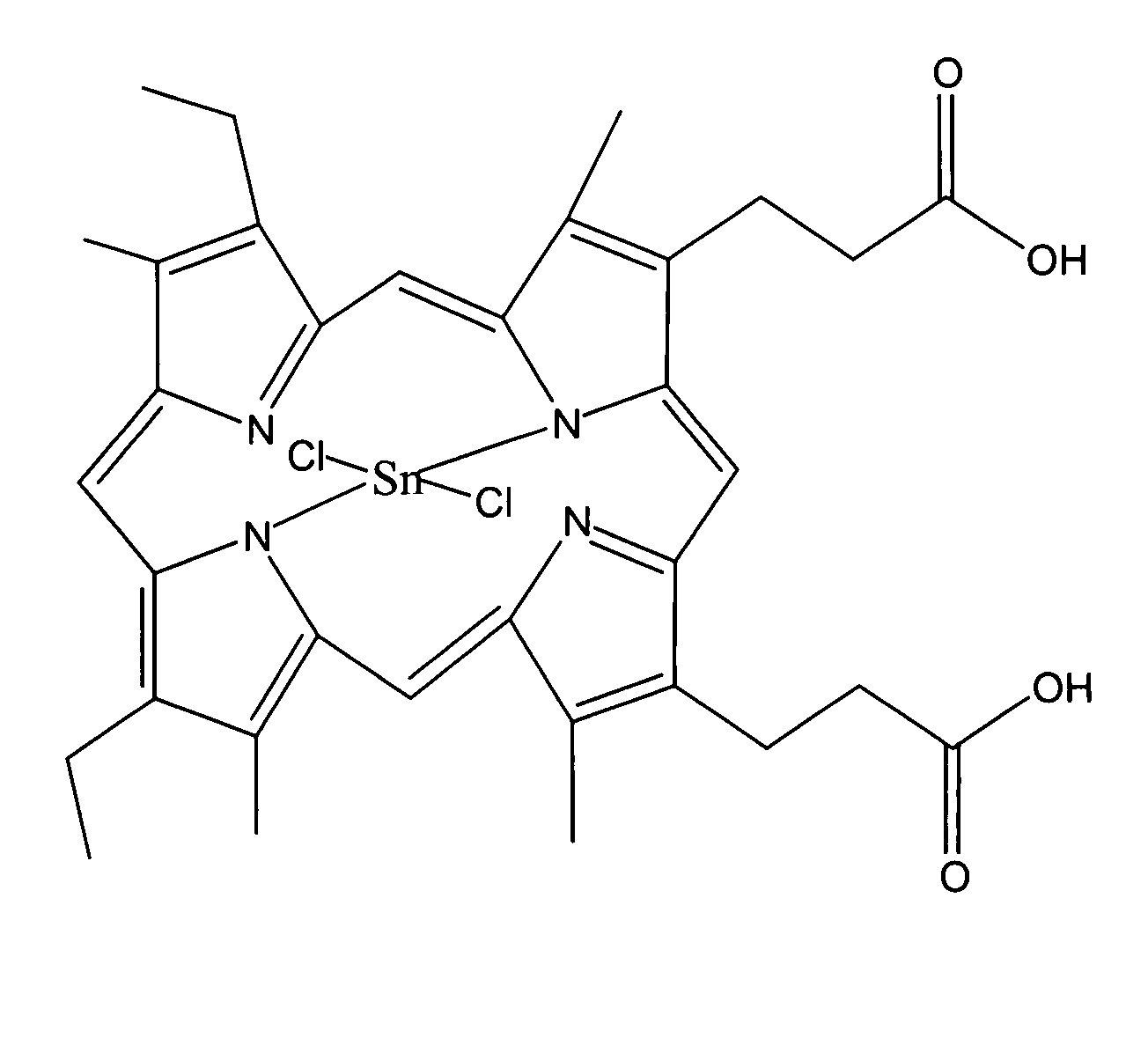
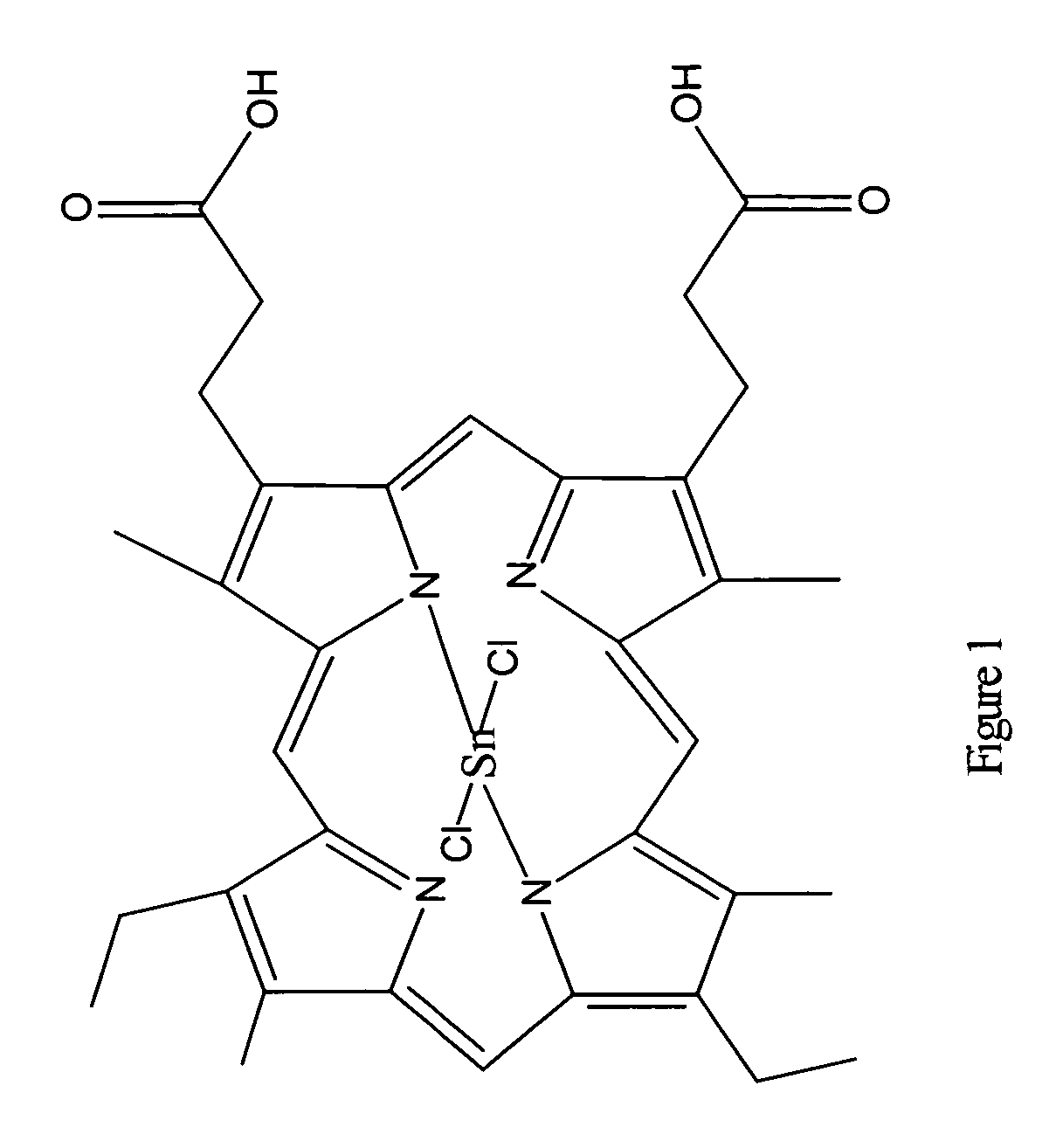
[0038]Preparation of Substantially Pure Tin Mesoporphyrin Chloride (Tin (IV) Mesoporphyrin IX Dichloride or Stannsoporfin).
[0039]A dark 1000 ml three-necked, round-bottom flask equipped with a mechanical stirrer, condenser, bubbler, and an aeration tube was charged with 30.0 g mesoporphyrin IX formate, 34.5 g tin (II) chloride, 7.1 g ammonium acetate, and 600 ml acetic acid. The suspension was stirred at 20-25° C. for 30 minutes. Mesoporphyrin IX formate and tin mesoporphyrin as well as all reaction intermediates are reportedly light sensitive materials therefore care was taken throughout this entire procedure to minimize the exposure of the reaction to light.
[0040]The reaction was warmed to reflux, with aeration, for 3 to 4 hours. The reaction was shown to be stable at 110-115° C. for up to 48 hours. Once complete, the reaction mixture was cooled to 60-70° C. and 300 ml water was added while cooling to 20-25° C. over 60 minutes. The suspension was filtered under reduced pressure. T...
example 3
[0041]Further Purification of Crude, Substantially Pure Tin (IV) Mesoporphyrin Chloride (Tin (IV) Mesoporphyrin IX Dichloride or Stannsoporfin).
[0042]A darkened, 250 ml, one-neck, round-bottom flask equipped with a magnetic stirbar and nitrogen purge was charged with: 10.0 g tin (IV) mesoporphyrin chloride (tin (IV) mesoporphyrin IX dichloride), 125 ml water, and 4 ml 28% ammonium hydroxide, a sufficient amount of ammonium hydroxide to adjust the pH to 9.0-10.0. The suspension was stirred at 20-25° C. for 20-30 minutes to effect dissolution. As tin (IV) mesoporphyrin is light sensitive, dark conditions were maintained throughout this reaction sequence.
[0043]The flask was charged with 0.5 g Darco KB, and a 1.5 g Celite. The dark suspension was stirred at 20-25° C. for 1 hour. The suspension was filtered under reduced pressure through a bed of celite using a 5.5 cm Buchner funnel. The flask and filtercake were rinsed with 2×10 ml water. A dark, 1L, one-neck, round-bottom flask equippe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com