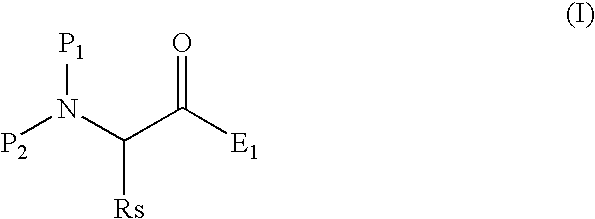
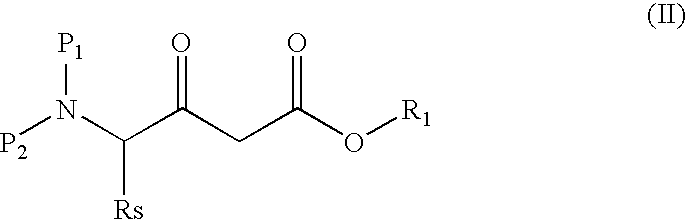
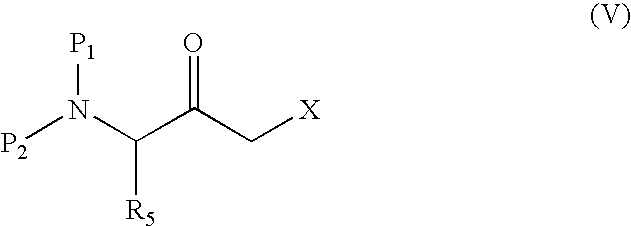Process for producing 3-amino-2-oxo-1-halogenopropane derivatives
a technology of halogenopropane and halogenomethyl anion, which is applied in the preparation of carbamic acid derivatives, organic chemistry, chemical production, etc., can solve the problems of unstable halomethyl anion use, and limited industrialization of this method, and achieves the effect of easy conversion into -aminoalcohol derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
production example 1
Production of N,N-dibenzyl-L-phenylalanine benzyl ester (Ia)
[0070]Twenty-five grams (151.3 mmol) of (L)-phenylalanine and 66.67 g (482.4 mmol) of potassium carbonate were dissolved in 100 ml of water, and 57.51 g (454.3 mmol) of benzyl chloride were added thereto. The mixture was heat-stirred at 95° C. for 19 hours. After the reaction mixture was cooled to room temperature, 67 ml of n-heptane and 50 ml of water were added thereto. The organic layer was washed twice with 50 ml of a mixture of methanol and water at a volume ratio of 1:2 and was then dried over anhydrous sodium sulfate. The dried substance was filtered and concentrated to give 61.64 g (90%, 121.8 mmol) of the above-mentioned compound (Ia) in a yield of 84.7%.
[0071]1H-NMR(300 MHz, CDCl3) δ:3.00 (dd,1H), 3.14(dd,1H), 3.53(d,2H),3.71(t,1H),3.92(d,2H),5.12(d,1H),5.23(d,1H), 6.99-7.40(m,20H); Mass spectrum (FAB) 436(MH+)
production example 2
Production of N,N-dibenzyl-L-phenylalanine p-nitrophenyl ester (1b)
[0072]N,N-dibenzyl-L-phenylalanine hydrochloride (7.64 g, 20.0 mmol) was added to 50 ml of chloroform, and 20.0 ml of 10% aqueous ammonia were added dropwise to the suspension for neutralization. The organic layer was separated, washed with 20 ml of water, then dried over magnesium sulfate, and filtered. The filtrate was concentrated, the resulting residue was dissolved in 50 ml of chloroform, and 2.89 g (20.4 mmol) of p-nitrophenol and 4.13 g (20.0 mmol) of N,N′-dicyclohexylcarbodiimide were added to the solution in this order while being cooled with ice. The mixture was reacted overnight. To the reaction solution were added 30 ml of ethyl acetate, and the N,N′-dicyclohexylurea which precipitated was removed by filtration. The filtrate was washed with a 10% potassium carbonate aqueous solution. The organic layer was separated, and concentrated. The resulting residue was redissolved in 30 ml of ethyl acetate, and the...
example 1
Production of (4S)-4-(N,N-dibenzylamino)-4-benzyl-3-oxobutanoic acid tert-butyl ester (IIa)
[0074]A solution (2.0M) (24 ml, 48 mmol) of LDA in heptane, THF and ethyl benzene was dissolved in 64 ml of anhydrous THF, and the mixed solution was cooled to −53° C. in an argon atmosphere. To this solution was added dropwise a solution of 5.8 g (50 mmols) of tert-butyl acetate in 12 ml of THF for approximately 15 minutes, while maintaining the temperature of from −45° C. to −50° C. After the completion of the dropwise addition, the mixture was stirred at −53° C. for 1 hour. Subsequently, a solution of 7.2 g (15 mmols, purity 90%) of N,N-dibenzyl-L-phenylalanine benzyl ester (Ia) in 8 ml of THF was added dropwise thereto for approximately 15 minutes while maintaining the temperature at from −48° C. to −52° C. After the completion of the dropwise addition, the reaction temperature was raised to −5° C. After three hours, a solution of 16.5 g of citric acid in 50 ml of water was added to the re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com