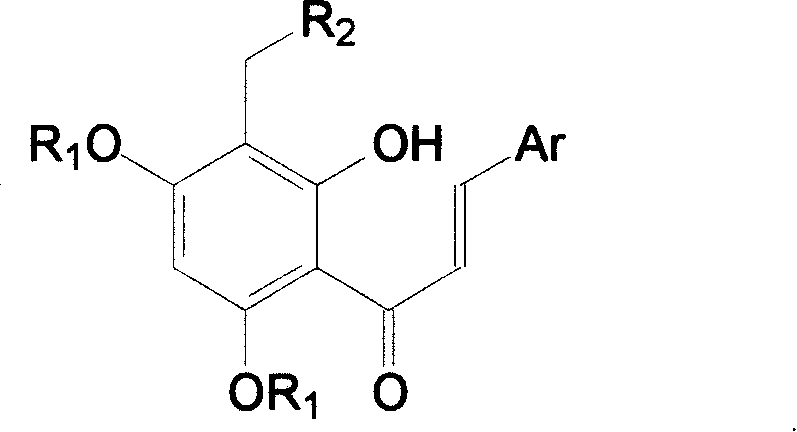
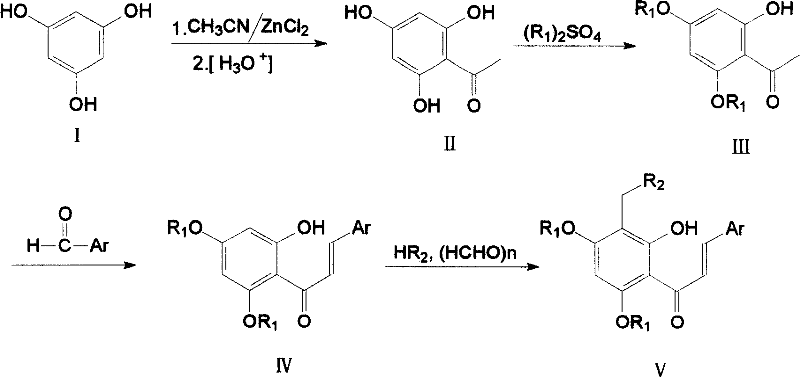
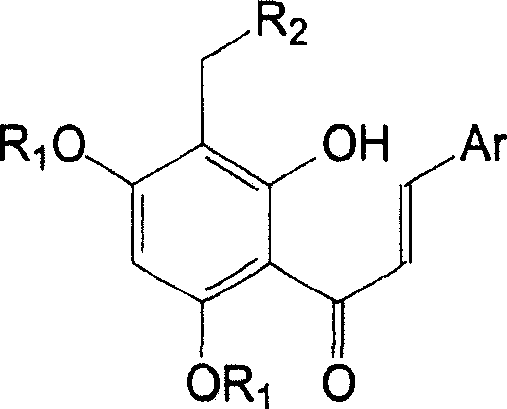
Tetra substituted chalcone derivative and preparing method and use
A chalcone derivative and disubstitution technology, applied in the field of synthesis of organic compounds, can solve problems such as the mechanism of action that has not been further clarified, and achieve the effects of low production cost, mild reaction conditions, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1. 2-Hydroxy-4,6-dimethoxy-acetophenone (III): prepared by referring to the literature method (K.Y.T.Juntend et al, EP 0292576).
[0021] M.p.77-78℃
[0022] 1 HNMR (δ, CDCl 3 ): 14.06(s, 1H), 6.05(d, 1H, J=2.4Hz), 5.92(d, 1H, J=2.4Hz), 3.90(s, 3H), 3.62(s, 3H), 2.63(s , 3H)
Embodiment 2
[0023] Example 2, 2'-Hydroxy-4', 6'-dimethoxy-2-chloro-chalcone (IV): Refer to the literature method (Xiaoyong Bu et al, Synthesis, 1997, 11, 1246-1248) be made of.
[0024] M.p.123-124℃
[0025] 1 HNMR (δ, CDCl 3 ): 14.20(s, 1H), 8.12-8.16(d, 1H, J=15.6Hz), 7.85-7.89(d, 1H, J=15.2Hz), 7.68-7.70(m, 1H), 7.42-7.44( m, 1H), 7.26-7.31(m, 2H), 6.10-6.11(d, 1H, J=2.4Hz), 5.95-5.96(d, 1H, J=2.4Hz), 3.90(s, 3H), 3.84 (s, 3H).
Embodiment 3
[0026] Example 3, 2'-hydroxyl-3'-(4-methylpiperazin-1-yl-methyl)-4',6'-dimethoxy-2-chloro-chalcone (Va):
[0027] Compound IV 1.59g (4.99mmol), formaldehyde aqueous solution 1.24g (15.30mmol), N-methylpiperazine 1.10g (10.98mmol), methanol 12.5ml and hydrochloric acid were added dropwise into the reaction flask, and refluxed for 2h. Cool, recover the solvent under reduced pressure, dilute with water, basify, extract with dichloromethane, wash with saturated sodium chloride, dry over anhydrous sodium sulfate, recover the solvent under reduced pressure, column chromatography (eluent: petroleum ether: ethyl acetate: Triethylamine=200ml:400ml:9.6ml) to obtain 1.17g of yellow solid, yield 56%. m.p.146-148°C.
[0028] 1 HNMR (δ, CDCl 3 ): 7.96-8.00(d, 1H, J=15.6Hz), 7.67-7.70(m, 1H), 7.38-7.42(d, 1H, J=15.6Hz), 7.39-7.41(m, 1H), 7.26- 7.29(m, 2H), 6.00(s, 1H), 3.86(s, 3H), 3.85(s, 3H), 3.71(s, 2H), 2.60-2.61(m, 4H), 2.44-2.45(m, 4H), 2.26(s, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com