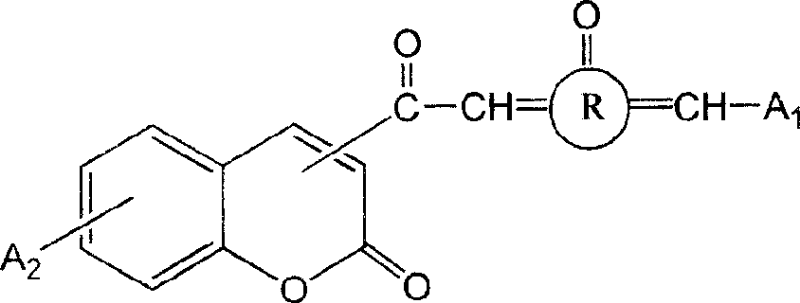
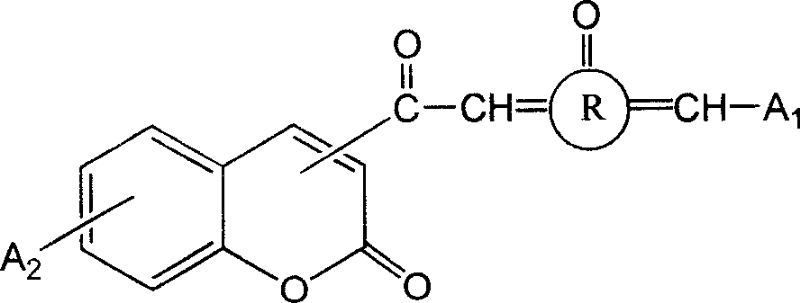
3- or 4- carbonyl substituted coumarin connected with naphthenones and its synthesis method and use
A technology of cycloalkane ketone and coumarin ketone, which is applied in the field of visible light sensitizing dyes, can solve the problem of wide absorption band coverage and achieve the effect of wide absorption band
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0113] Synthesis of coumarin ketones (CP-KETOCOU) substituted with 3 or 4 carbonyl groups linked by cyclopentanone
[0114](1) Put 40 millimoles of cyclopentanone and 10 millimoles of p-dimethylaminobenzaldehyde into the flask, add 7 milliliters of absolute ethanol and 10 milliliters of water, add 0.5 milliliters of hexahydropyridine dropwise, at room temperature The reaction was conducted at 25°C for 5 hours, the orange precipitate was filtered, and column chromatography was performed with 0.2wt% ethanol / chloroform to obtain semi-p-dimethylaminobenzylidene cyclopentanone dye with a yield of 85%.
[0115] (2) (Refer to the literature "Journal of Medicinal Chemistry" 1983, volume 31, page 3014, ChemPharm Bull, 1983, 31, 3014) 10 millimoles of 4-acetyl-5-pyrrolyl coumarin, 12 millimeters Place moles of selenious acid in the flask, add 50 ml of xylene and heat to dissolve, reflux for 10 hours, hot filter to remove the gray-black selenium, let the filtrate stand and cool to precipitat...
Embodiment 2
[0118] Synthesis of coumarin ketones (CHE-KETOCOU) substituted with 3 or 4 carbonyl groups linked with cyclohexanone
[0119] (1) Put 30 millimoles of cyclohexanone and 10 millimoles of acetaldehyde into the flask, add 20 milliliters of absolute ethanol and 5 milliliters of water, drop 2 milliliters of 10wt% NaOH aqueous solution, and react at 60°C for 24 hours , Filter the orange precipitate, and perform column chromatography with 0.2wt% ethanol / chloroform to obtain half 2-ethylcyclohexanone dye with a yield of 75%.
[0120] (2) (Refer to the literature "Journal of Medicinal Chemistry" 1983, Volume 31, page 3014, ChemPharm Bull, 1983, 31, 3014) (Refer to the literature "Journal of Medicinal Chemistry", ChemPharm Bull, 1983, 31, 3014) 10 millimoles of 3-acetyl-7-methoxycoumarin, 15 milliliters Place moles of selenous acid in the flask, add 60 ml of ethanol to heat to dissolve, reflux for 15 hours, hot filter to remove the gray-black selenium, leave the filtrate to stand and cool t...
Embodiment 3
[0123] Synthesis of coumarin ketones (CHP-KETOCOU) substituted with 3 or 4 carbonyl groups linked by cycloheptanone
[0124] (1) Put 20 millimoles of cycloheptanone and 10 millimoles of benzaldehyde into the flask, add 20 milliliters of absolute ethanol and 2 milliliters of water, drop 3 milliliters of 10wt% KOH aqueous solution, and react at 65°C for 36 hours , Filter the orange precipitate, and perform column chromatography with 0.2wt% ethanol / chloroform to obtain the hemibenzylidene cycloheptanone dye with a yield of 65%.
[0125] (2) (Refer to the literature "Journal of Medicinal Chemistry" 1983, Volume 31, page 3014, ChemPharm Bull, 1983, 31, 3014) (Refer to the literature "Journal of Medicinal Chemistry", ChemPharm Bull, 1983, 31, 3014) 10 millimoles of 3-methyl-6-phenyl coumarin, 18 millimoles Place selenous acid in a flask, add 70 ml of 1,4-dioxane and heat to dissolve, reflux for 24 hours, hot filter to remove gray-black selenium, let the filtrate stand and cool to precip...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com