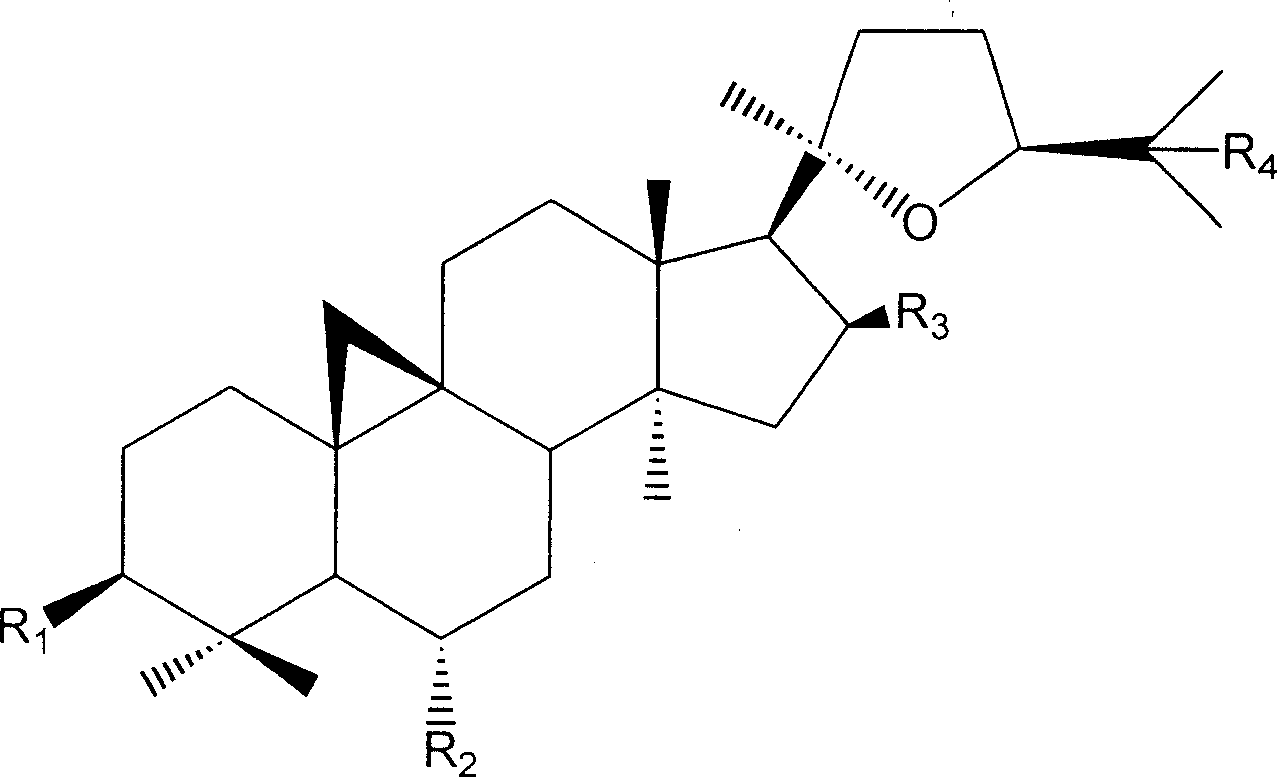
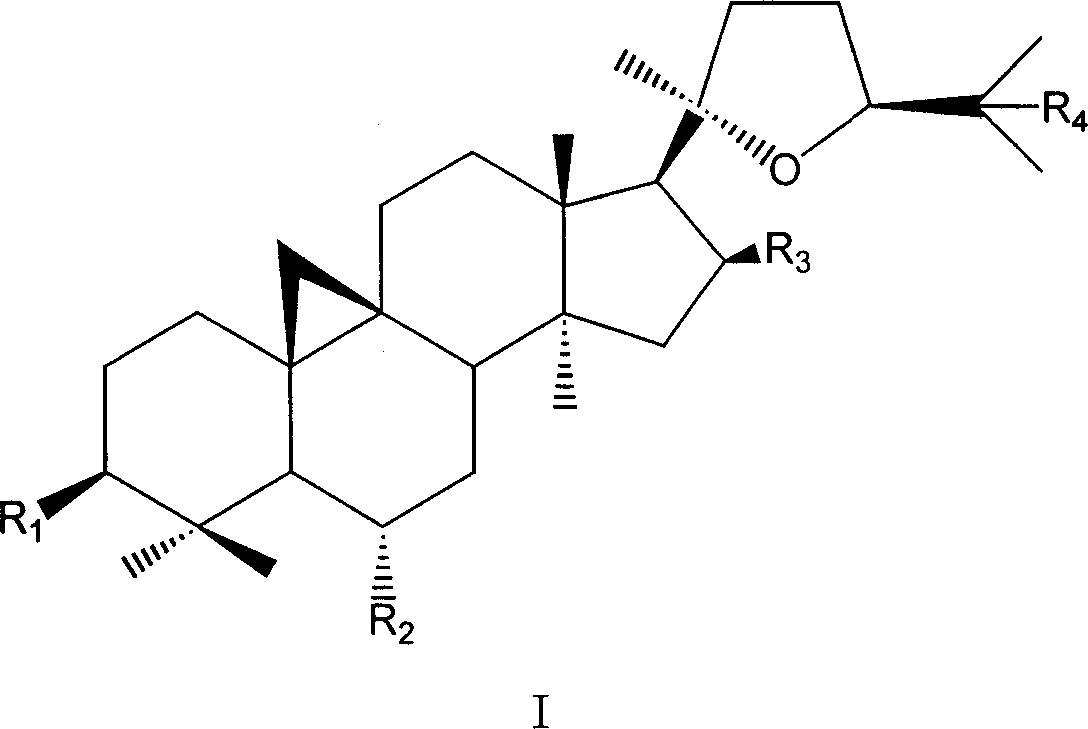
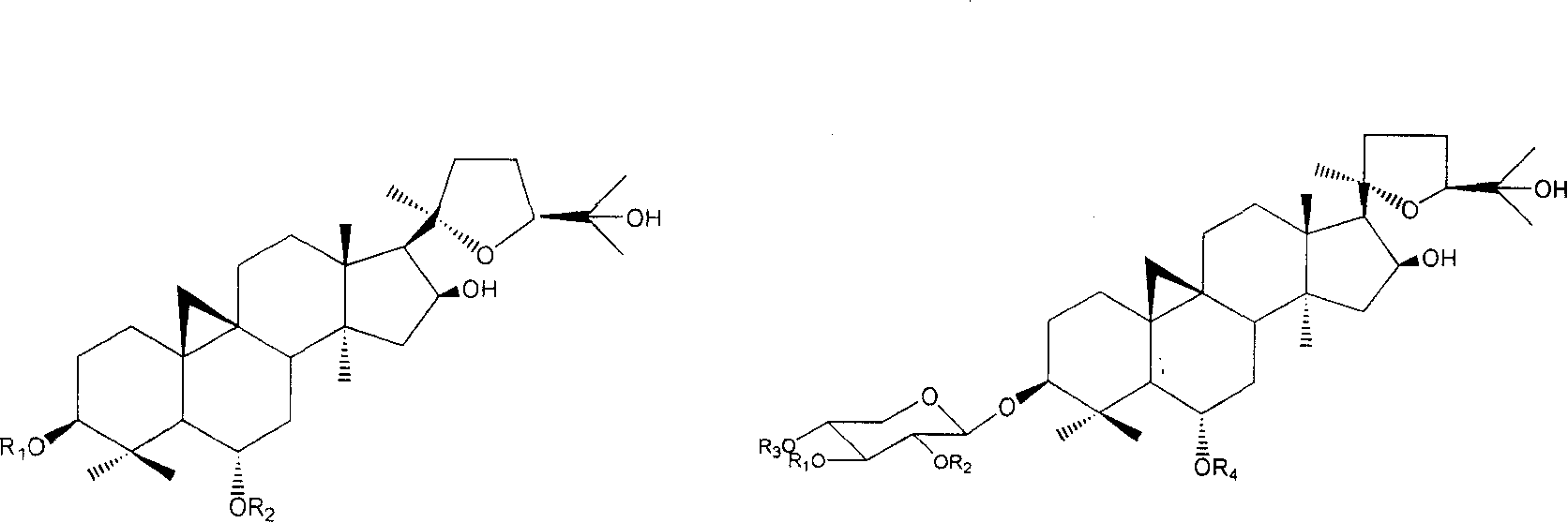
Derivative of cyclo membranousol kind and application thereof
A technology of cycloastragenol and derivatives, which is applied in the field of medicine, can solve the problems of application limitation, irritating reaction, dosage limitation, etc., and achieve the effect of improving bioavailability and water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0081] In the preparation method described herein, cycloastragenol or its naturally derived derivative astragaloside IV is used as a raw material.
[0082] Among them, cycloastragenol is obtained by hydrolyzing total saponins of astragalus through known conventional hydrolysis technology (enzyme hydrolysis, acid hydrolysis); astragaloside IV is prepared according to the patented technology with application number 200410007963.0.
Embodiment 1
[0083] The preparation of embodiment 1 cycloastragenol phosphate ester salt
[0084] a. Add 1g of cycloastragenol, 3g of dibenzyl phosphate, 2.7g of triphenylphosphine and 200ml of ethyl acetate in the reaction flask, cool to 15°C under stirring, add dropwise 1g of diethyl azodicarboxylate (DEAD ), lasted about 30 minutes, the dropwise addition was completed, continued to stir for 1 hour, raised the temperature to 35 ° C, and kept the reaction for 5 hours. After the reaction was completed, the ethyl acetate layer was washed with aqueous sodium bicarbonate and dried overnight over anhydrous sodium sulfate. Ethyl acetate was evaporated under reduced pressure to obtain an oil. After silica gel column chromatography and dichloromethane / methanol solvent system elution and separation, the dibenzyl phosphate of cycloastragenol was obtained.
[0085] b. Debenzylation: take 0.5 g of cycloastragenol dibenzyl phosphate, add 50 ml of methanol, add 0.2 g of 10% palladium carbon, ventilat...
Embodiment
[0089] The preparation of embodiment 2 cycloastragenol 3-O-sulfonate salt
[0090] a. Take 6g of cycloastragenol that has been ground through a 40-mesh sieve, add 300ml of absolute ethanol, cool to 5°C-10°C under stirring, slowly add 50ml of concentrated sulfuric acid dropwise, after the addition is complete, heat up to 20°C-25°C, Insulated and stirred for 48 hours. After the reaction is complete, add 300ml of absolute ethanol, take an ice-water bath, under stirring, adjust the pH value to 7 with 40% sodium hydroxide, and let stand for 24 hours. Add an appropriate amount of activated carbon, filter, and evaporate the filtrate under reduced pressure to obtain the sulfonated cycloastragenol.
[0091] b. Separation and purification: Dissolve 5 g of the sulfonated cycloastragenol in 25 ml of water, filter and apply to a D101 macroporous resin column, and elute with water, 10%, 30%, 50%, 70%, and 90% ethanol in sequence (TLC trace). The combined Rf values are comparable to the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com