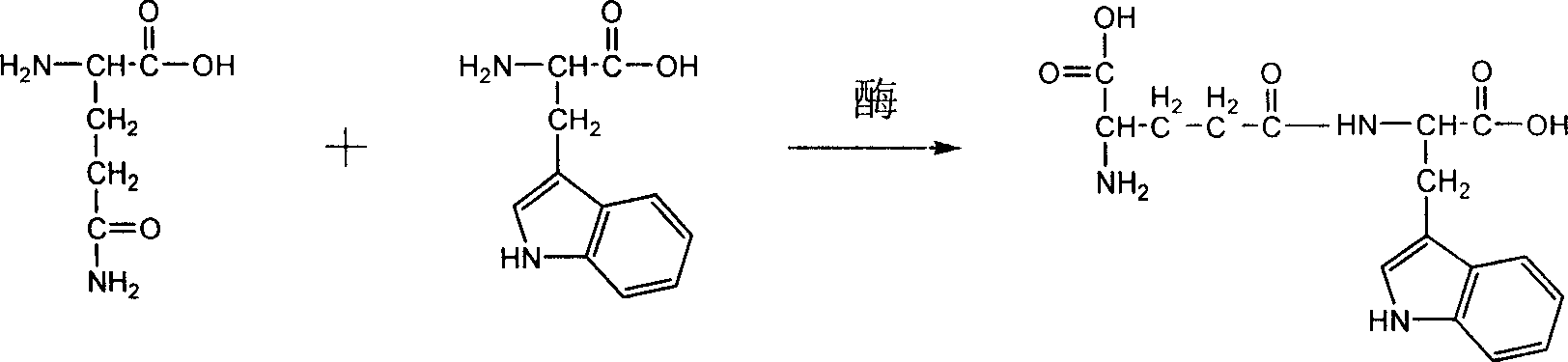
Process of synthesizing gamma-D-glutamine acyl L-tryptophane by enzyme method
A technology of enzymatic synthesis and tryptophan, which is applied in fermentation and other directions, can solve the problems of high operating costs, side reactions, and low conversion rate, and achieve high recovery, simple method, and good purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0037] Preparation Example 1: Preparation and Purification of γ-Glutamyl Transpeptidase
[0038] Take 15g of sucrose, 30g of corn steep liquor, 15g of peptone, K 2 HPO 4 15g, MgSO 4 0.5g, pH 7.5, add tap water to make 1L liquid culture medium, prepare 3.5L according to this recipe, put it into a 5L fermenter and put it into a 0.1MPa high-pressure steam sterilization for 25 minutes for later use. At the same time, divide 50mL of medium into 500mL, add 8 layers of gauze, wrap it in kraft paper, and sterilize it together with the fermenter for later use.
[0039] After taking out the bacterial species preserved in the refrigerator—Bacillus subtillis NX-2 (preservation number is CGMCCNo.0833), connect it to fresh slant medium (slant medium (g / L): peptone 10, beef extract 3. On NaCl5, agar 20), activate the culture for 24 hours, insert the seed medium (seed medium (g / L): glucose 15, corn steep liquor 10, peptone 10, K 2 HPO 4 2. MgSO 4 0.25) shake culture at 33° C. and 220 r...
Embodiment 1
[0042] Get the gamma-glutamyl transpeptidase 0.02U that preparation example 1 obtains, add 0.0292g gamma-D-glutamine, L-tryptophan 0.0489g, Tris (trishydroxymethylaminomethane)-HCl (pH9.0 , 0.2mol / L) 20mL, after mixing evenly, stir and react for 5h, and the reaction temperature is 38°C. After the reaction finishes, analyze and detect with high performance liquid chromatography system (chromatographic separation condition: chromatographic column is Kromasil KR100-5C18 post, and detection wavelength is 220nm, mobile phase is 75%, 20mmol / L KH 2 PO 4 , 25% methanol; flow rate: 1 mL / min), the conversion rate of γ-D-glutamyl-L-tryptophan was 58%, and the purity was 98%.
[0043] After the reaction, the reaction solution was micro-filtered to remove enzymes, and then KTA TM The explorer 100 protein purification instrument was used for separation and purification. The separation and purification conditions were as follows: the chromatographic column was a reversed-phase C18 column...
Embodiment 2
[0045] Get 0.5U of γ-glutamyl transpeptidase obtained in Preparation Example 1, add 0.0584g γ-D-glutamine, 0.122g L-tryptophan, NaHCO 3 -NaOH (pH10.5, 0.1mol / L) 20mL, after mixing evenly, stir and react for 6h, the reaction temperature is 37°C. After the reaction finishes, analyze and detect with high performance liquid chromatography system (chromatographic separation condition: chromatographic column is Kromasil KR100-5C18 post, and detection wavelength is 220nm, mobile phase is 75%, 20mmol / L KH 2 PO 4 , 25% methanol; flow rate: 1 mL / min), the conversion rate of γ-D-glutamyl-L-tryptophan was 65%, and the purity was 98.4%.
[0046] After the reaction, the reaction solution was micro-filtered to remove enzymes, and then KTA TM The explorer 100 protein purification instrument was used for separation and purification. The separation and purification conditions were as follows: the chromatographic column was a reversed-phase C18 column, the detection wavelength was 214nm, the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com