Method for synthesizing anticancer compound CA4
A synthesis method, CA4 technology, applied in the field of synthesis of anticancer compound CA4, can solve the problems of increasing synthesis steps and production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
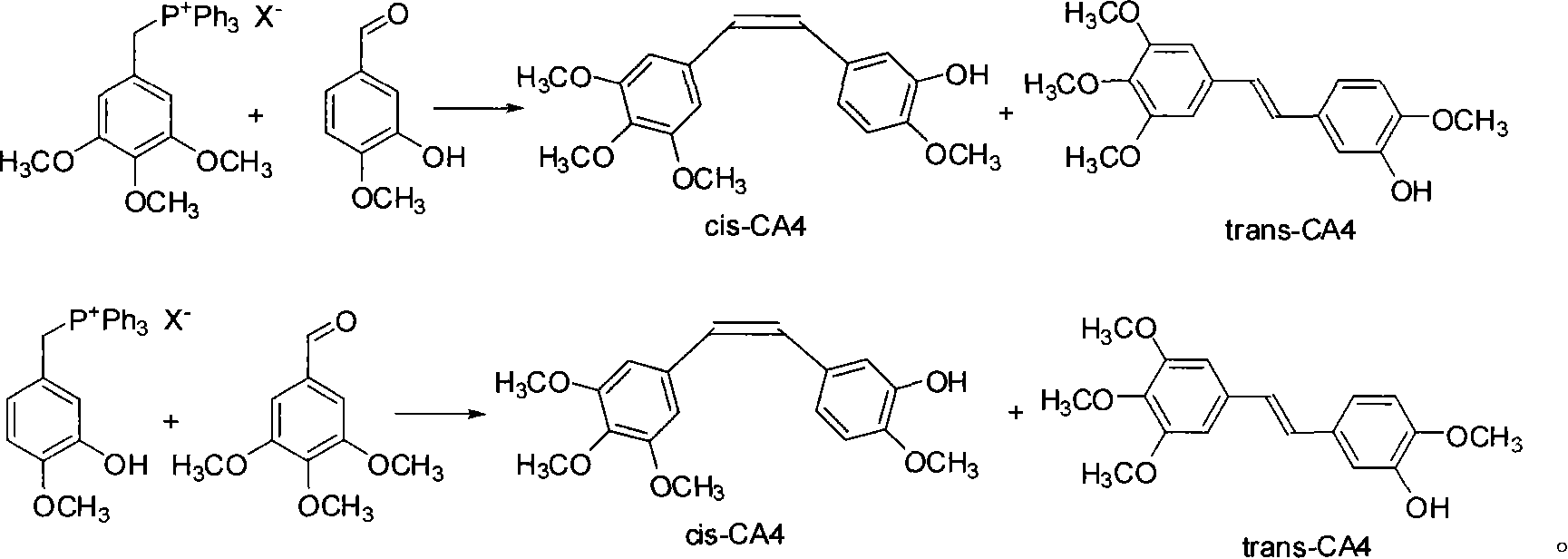
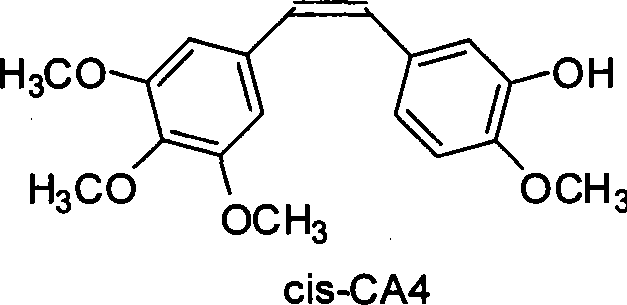
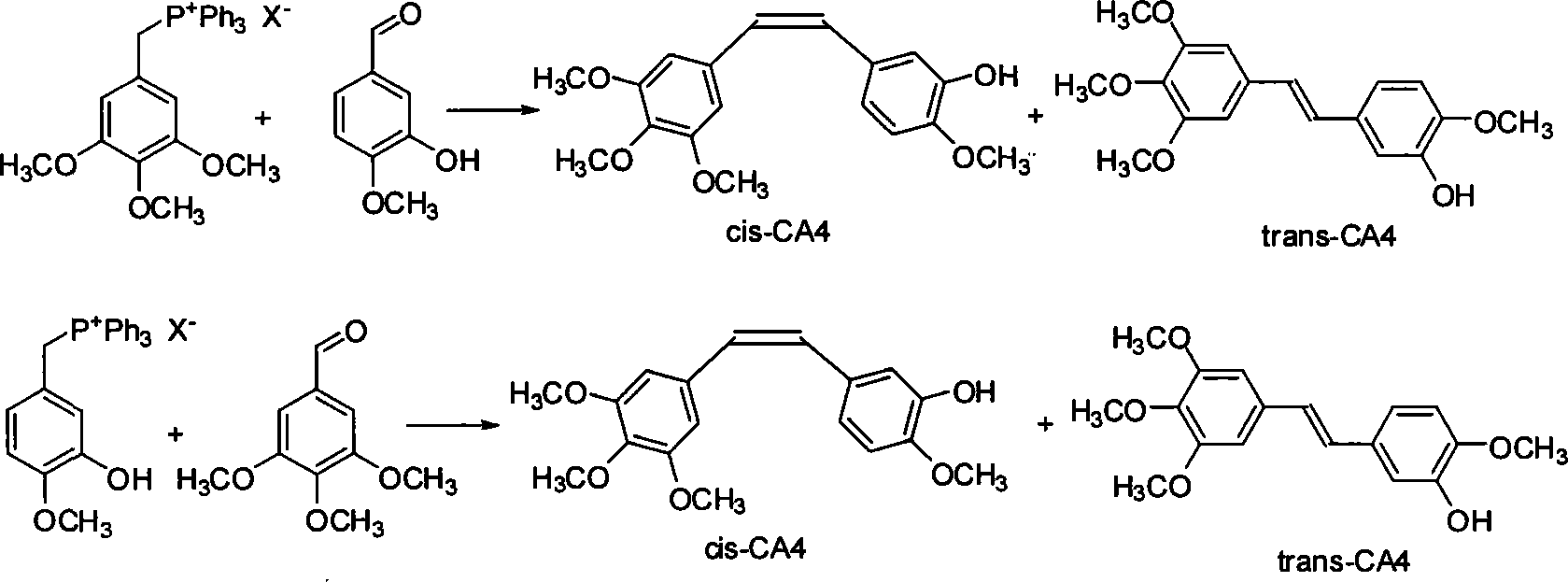
[0017] Add 1.654g 3,4,5-trimethoxybenzyltriphenylphosphonium bromide salt, 0.400g 3-hydroxyl-4-methoxybenzaldehyde, 0.545g potassium carbonate, 35mL ethanol in a 100mL flask, Under protection, it was heated to reflux for 8 h, and TLC (developing agent chloroform:ethyl acetate=8:1) showed that the reaction was complete. Spin off ethanol, add ethyl acetate, acidify with 20mL of 5% hydrochloric acid, wash twice with water, wash the organic phase once with saturated sodium bisulfite, dry the organic layer with anhydrous sodium sulfate, filter off sodium sulfate, and reduce the reaction solution to Concentrate under pressure to obtain an oil. The crude product was separated by silica gel column chromatography (petroleum ether: ethyl acetate = 5:1 as eluent), and the fraction containing CA4 was collected to obtain 0.800 g of the product with a yield of 96%. Utilize HPLC to carry out the qualitative and separation of components (wavelength: 254nm, mobile phase: methanol / water=60 / 40)...
Embodiment 2
[0019] In the flask of 100mL, add 1.654g 3,4,5-trimethoxybenzyltriphenylphosphonium bromide salt, 0.400g 3-hydroxyl-4-methoxybenzaldehyde, 0.220g potassium hydroxide, 35mL ethanol, in Under the protection of nitrogen, it was heated to reflux for 6 h, and TLC (developing solvent: chloroform: ethyl acetate = 8: 1) showed that the reaction was complete. Spin off ethanol, add ethyl acetate, acidify with 20mL of 5% hydrochloric acid, wash twice with water, wash the organic phase once with saturated sodium bisulfite, dry the organic layer with anhydrous sodium sulfate, filter off sodium sulfate, and reduce the reaction solution to Concentrate under pressure to obtain an oil. The crude product was separated by silica gel column chromatography (petroleum ether: ethyl acetate = 5:1 as the eluent), and the fraction containing CA4 was collected to obtain 0.803 g of the product with a yield of 97%. Utilize HPLC to carry out the qualitative and separation of components (wavelength: 254nm,...
Embodiment 3
[0021]Add 1.654g 3,4,5-trimethoxybenzyltriphenylphosphonium bromide salt, 0.400g 3-hydroxyl-4-methoxybenzaldehyde, 0.545g potassium carbonate, 35mL water in a 100mL flask, Under protection, it was heated to reflux for 10 h, and TLC (developing agent chloroform:ethyl acetate=8:1) showed that the reaction was complete. Add ethyl acetate for extraction, acidify with 20 mL of 5% hydrochloric acid, wash twice with water, wash the organic phase once with saturated sodium bisulfite, dry the organic layer with anhydrous sodium sulfate, filter off sodium sulfate, and concentrate the reaction solution under reduced pressure to obtain Oil. The crude product was separated by silica gel column chromatography (petroleum ether: ethyl acetate = 5:1 as eluent), and the fraction containing CA4 was collected to obtain 0.591 g of the product with a yield of 71%. Utilize HPLC to carry out the qualitative and separation of components (wavelength: 254nm, mobile phase: methanol / water=60 / 40), by inte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com