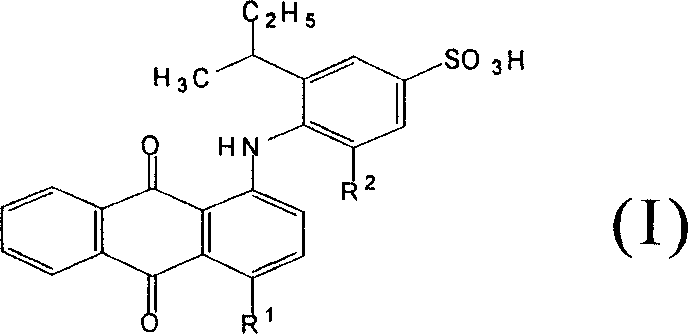
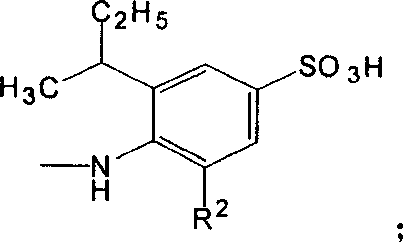
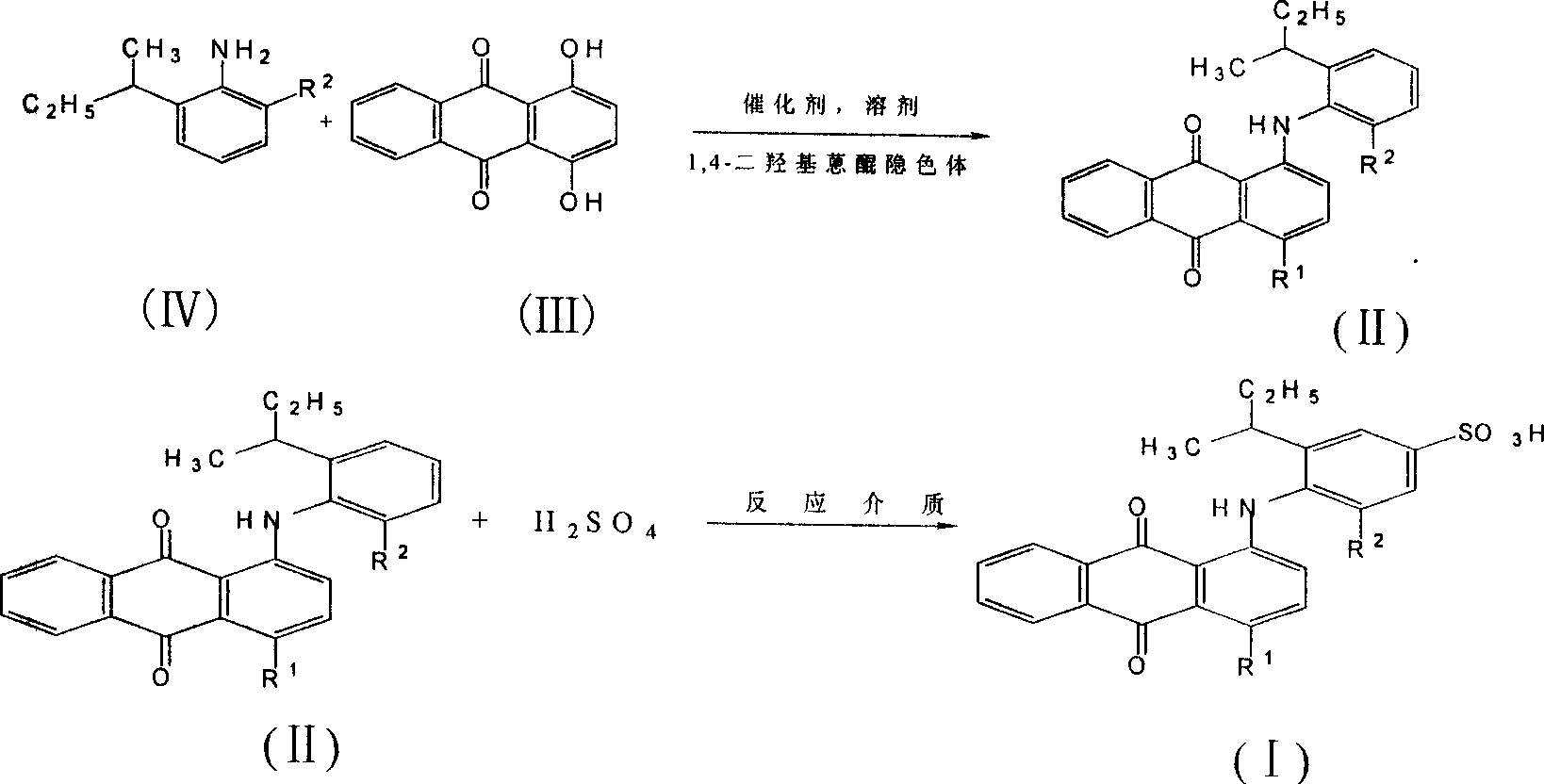
Acid anthraquinone dye and its prepn and application
An acid dye, anthraquinone type technology, applied in the field of dyes, can solve the problems of expensive dyes, limited application amount and high price, and achieve the effects of reducing dye costs, raw material costs, environmental protection and resource utilization.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0029] Example 1: Separation and purification of 2-ethyl-6-sec-butylaniline
[0030] Rectification: Add 600g of industrial waste oil produced by DEA production into a 1000ml three-necked bottle, add zeolite, and pass nitrogen gas through the bottom tube. Distillation conditions: the diameter of the rectification tower is 30mm, the stainless steel triangular packing, the packing height is 1100mm, the vacuum degree is 10mmHg, and the reflux ratio is 5:1. When the liquid phase temperature reached 137°C, reflux occurred, and the fractions at 141-144°C were collected to obtain 300 g of the main fraction, which was 2-ethyl-6-sec-butylaniline with a content of 95%. The analysis data is as follows: 1 H NMR (DCCl 3 , 300MHz) δ 0.89-0.94 (m, 3H, CH 3 ), 1.22-1.29 (m, 6H, CH 3 ), 1.54-1.71 (m, 2H, CH 2 ), 2.49-2.57 (m, 2H, CH 2 ), 2.65-2.68(m, 1H, CH); 6.73-6.7(m, 1H, Ar), 6.94-7.01(m, 2H, Ar), 3.64(s, 2H, NH 2 ); m / z: 177.
example 2
[0031] Example 2: Separation and purification of 2-methyl-6-sec-butylaniline
[0032] Rectification: Add 600g of industrial waste oil produced in the production of MEA into a 1000ml three-necked bottle, add zeolite, and pass nitrogen gas through the bottom tube. Distillation conditions: the diameter of the rectification tower is 30mm, the stainless steel triangular packing, the packing height is 1100mm, the vacuum degree is 10mmHg, and the reflux ratio is 5:1. When the liquid phase temperature reached 127°C, reflux occurred, and the fraction at 130-133°C was collected to obtain 268g of the main fraction, which was 2-methyl-6-sec-butylaniline with a content of 93.5%. The analysis data is as follows: 1 H NMR (DCCl 3 , 300MHz) δ 0.88-0.92 (m, 3H, CH 3 ), 1.21-1.26 (m, 3H, CH 3 ), 1.54-1.71 (m, 2H, CH 2 ), 2.39-2.47 (m, 3H, CH 3 ), 2.65-2.68 (m, 1H, CH); 6.73-6.77 (m, 1H, Ar), 6.92-6.98 (m, 2H, Ar), 3.64 (s, 2H, NH 2 ); m / z: 163.
example 3
[0033] Example 3: Synthesis of dye a:
[0034] Add 8.7g (0.0362mol) 1,4-dihydroxyanthraquinone, 3.7g (0.0152mol) 1,4-dihydroxyanthraquinone leuco, 1.5g boric acid, 40g 2-ethyl - 6-sec-butylaniline, stir and raise the temperature to 150°C, while removing the water brought in by the raw materials and produced by the reaction. The reaction is controlled by thin-layer chromatography: when the yellow and orange raw material spots basically disappear, the reaction is considered to have reached the end, stop the reaction, lower the temperature to 90°C, add 6g of potassium hydroxide, and pass through air to oxidize, until the purple color disappears completely , which is the end point of oxidation, slowly analyze the reaction solution into 120ml hot ethanol, then cool down, filter and wash to obtain the blue intermediate dye 1,4-di(2-ethyl-6-sec-butylanilino)anthracene Quinone 20.0g. The analysis data is as follows: 1 H NMR (DCCl 3 , 300MHz) δ 0.77-0.83 (m, 3H, CH 3 ), 1.01-1.17 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com