Synthesis method for levorotatory citirizine dihydrochloride
A levocetirizine and synthetic method technology, applied in the direction of organic chemistry, etc., can solve the problems of harsh reaction conditions, expensive reagents, poor stereoselectivity, etc., and achieve the effect of simple synthesis process, good optical purity, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
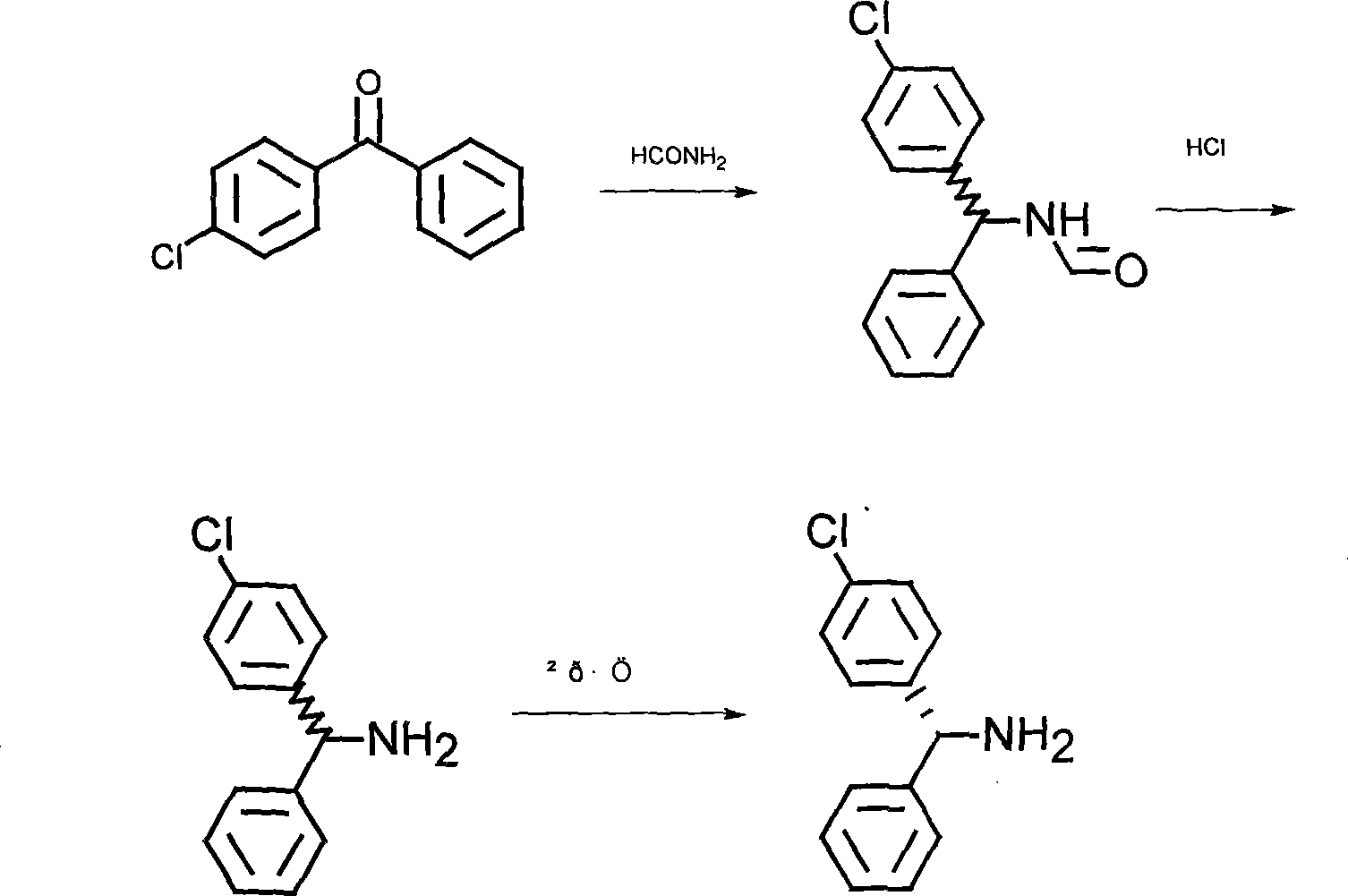
[0022] The synthesis of embodiment 1 mixed-rotation mono-p-chlorobenzhydrylamine
[0023] In a 3-liter reaction bottle, add 2 liters of formamide and 500 grams of mono-p-chlorobenzophenone, heat to 170-180°C, and react for 17-24 hours. After the reaction of mono-p-chlorobenzophenone is completed, After cooling, add it to 5 liters of water to form a yellow-white solid. After cooling for 4 to 5 hours, filter, wash with water, and directly hydrolyze without drying. Add the above solid to 900 ml of concentrated hydrochloric acid, add 440 ml of water, heat and reflux for 2 hours, place in the refrigerator to cool for 7 to 10 hours, filter and wash with water, decolorize the solid wet material with 5 liters of water, 30 to 50 grams of activated carbon, and recrystallize . After cooling for 7 to 10 hours, filter and dry 450 grams of diphenylmethylamine hydrochloride.
Embodiment 2
[0024] The preparation of embodiment 2 left-handed mono-p-chlorobenzhydrylamine
[0025] Mix 340 grams of mono-p-chlorobenzhydrylamine hydrochloride and add it to a mixed solvent of 2000 milliliters of methanol and 1000 milliliters of water, add 54 grams of NaOH, then add 250 grams of levorotatory o-chloromandelic acid, heat to dissolve, then stir and crystallize at room temperature , to obtain 250 grams of o-chloromandelate solids of L-mono-p-chlorobenzhydrylamine. The pH was adjusted to alkaline with NaOH solution, and then extracted three times with 2000 ml of ethyl acetate. The ethyl acetate was evaporated to dryness to obtain 120 g of L-mono-p-chlorobenzhydrylamine.
Embodiment 3
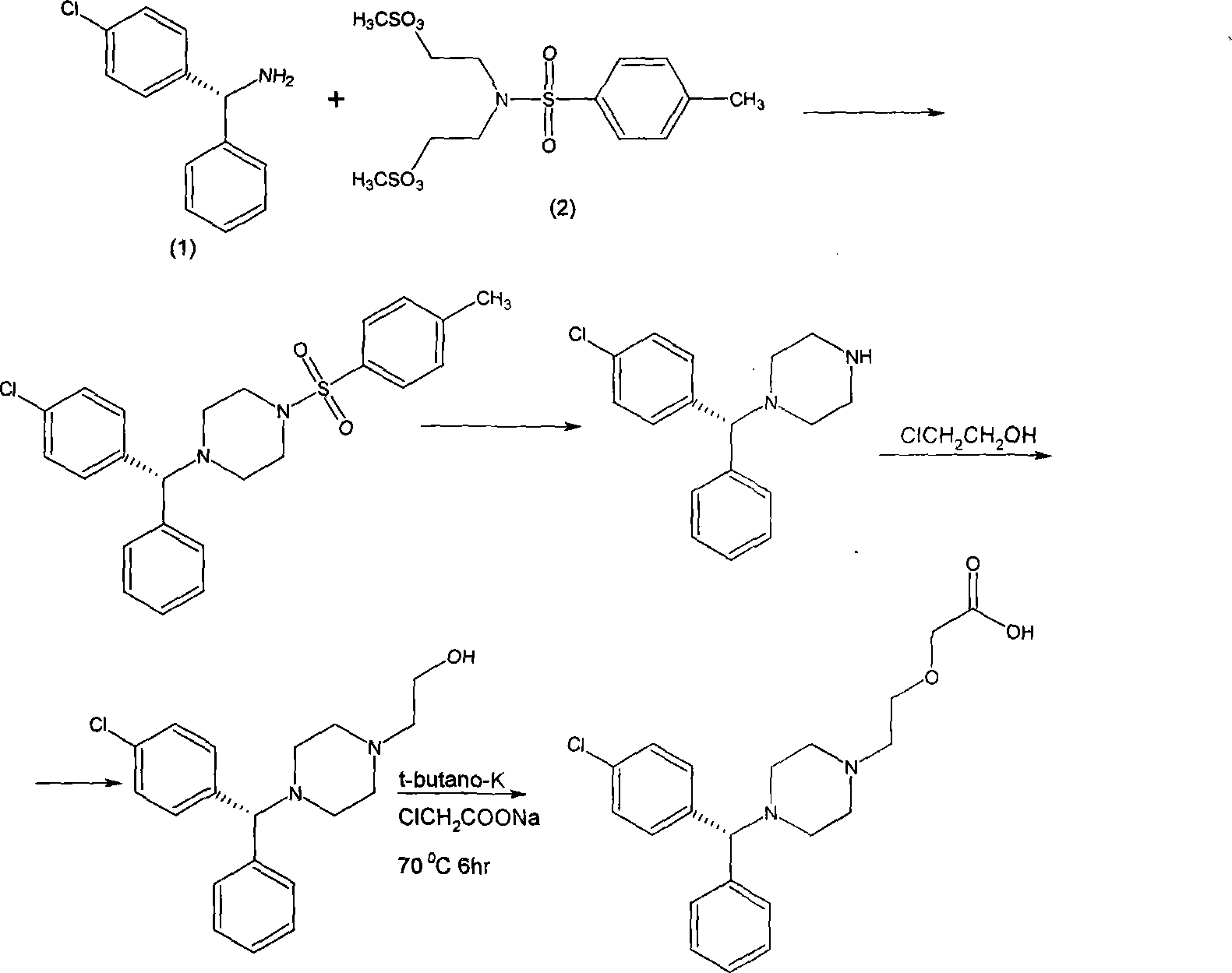
[0026] Example 3 Synthesis of left-handed 1-N-single p-chlorobenzhydryl-4-N-p-toluenesulfonylpiperazine
[0027] 120 grams of L-mono-p-chlorobenzhydrylamine, dissolved in 1200 milliliters of xylene, added 152 grams of N, N-dimethylsulfonate ethyl p-benzenesulfonamide, Na 2 CO 3 117.2 g, stirred and refluxed for 24 hours, separated the xylene organic phase, evaporated to dryness, added 200 ml of methanol, cooled and crystallized for 4 to 5 hours, filtered to obtain L-1-N-mono-p-chlorobenzhydryl-4 -N-p-toluenesulfonylpiperazine 90 g.
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com