Manganese or iron catalyzer of 8- hydroxyquinoline derivant of hexa-tooth coordination structure and uses of the same
A technology of hydroxyquinoline and iron catalysts, applied in the direction of organic compound/hydride/coordination complex catalysts, physical/chemical process catalysts, compounds of Group 7/17 elements of the periodic table, etc., to achieve high chemoselectivity, The effect of simple preparation and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
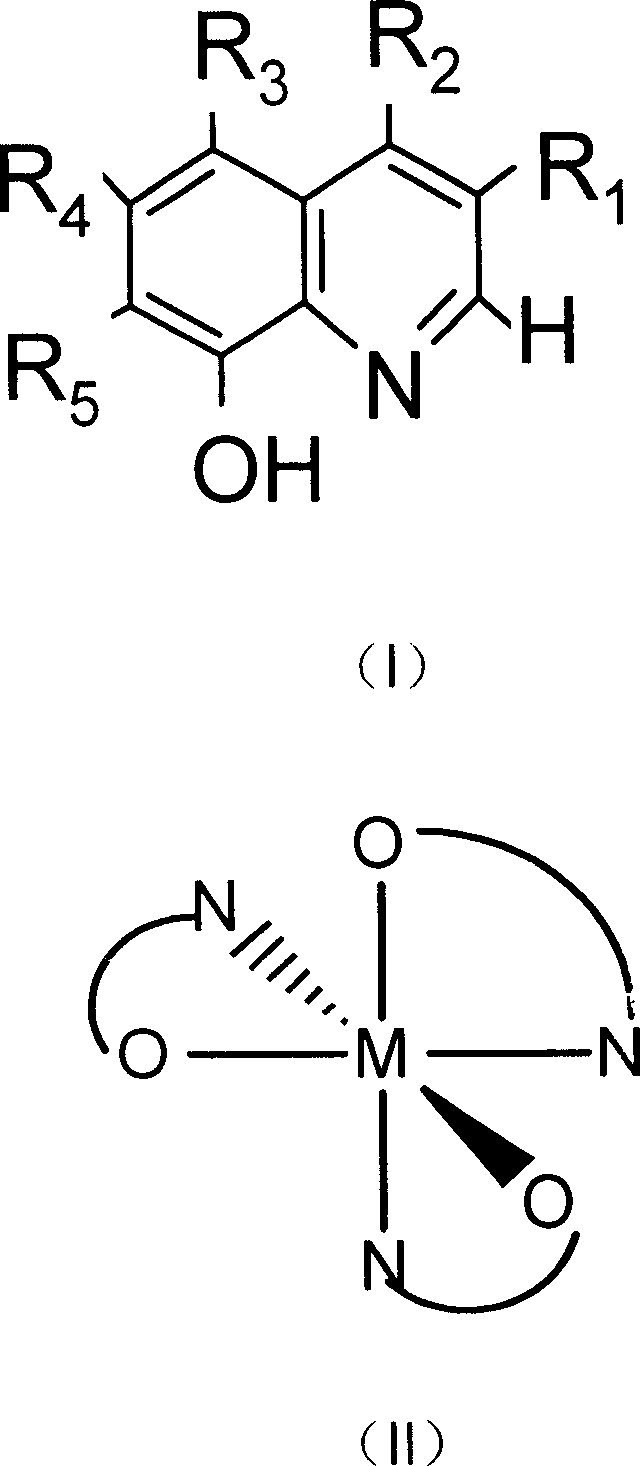
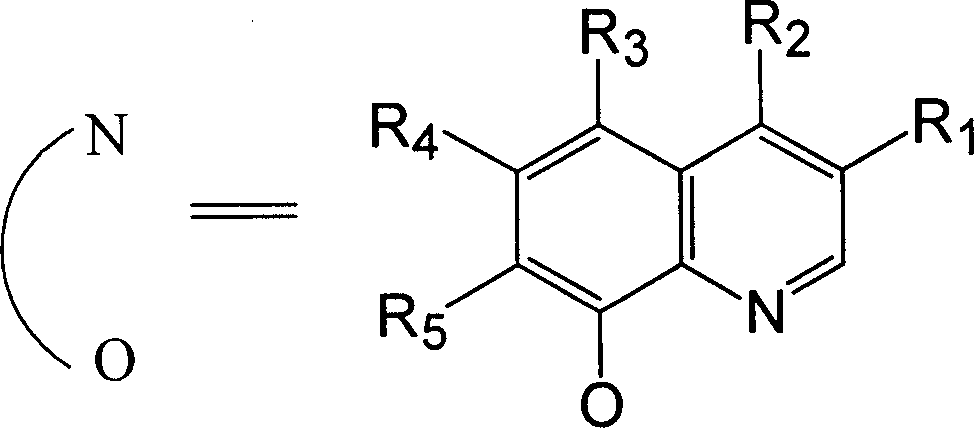
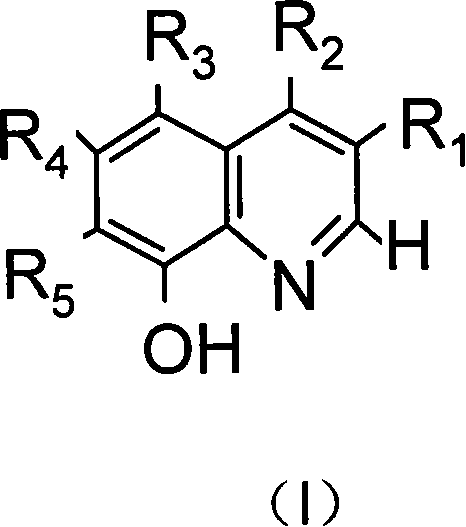
[0031] Embodiment 1: meet the synthesis of the manganese catalyst of general formula (II):
[0032] Dissolve 15 mmol of the ligand conforming to the general formula (I) in 30-50 ml of tetrahydrofuran, and add 1 mol / L manganese acetate or manganese chloride solution 5 ml dropwise under stirring. Then add 0.57 g (5 mmol) of 30% hydrogen peroxide, and then drop concentrated ammonia water to adjust the pH value of the solution to 7. Continue to stir or reflux at room temperature for 2-3 hours. Suction filtration, washing with ethanol, and drying to obtain the manganese catalyst of general formula (II). The product yield is about 92%.
Embodiment 2
[0033] Embodiment 2: meet the synthetic method two of the manganese catalyst of general formula (II):
[0034] Dissolve 15 mmol of the ligand conforming to the general formula (I) in 30-50 ml of tetrahydrofuran, and add 0.57 g (5 mmol) of 30% hydrogen peroxide under stirring. Then, concentrated ammonia water was added dropwise to adjust the pH value of the solution to 7 to obtain a solution A. Add 20 mL of 0.25 mol / L manganese acetate or manganese chloride ethanol solution to solution A dropwise under stirring. Continue to stir and reflux for 2 to 3 hours. Suction filtration, washing with ethanol, and drying to obtain the manganese catalyst of general formula (II). The product yield is about 94%.
Embodiment 3
[0035] Embodiment 3: meet the synthetic method of the iron catalyst of general formula (II):
[0036] Dissolve 15 mmol of the ligand conforming to the general formula (I) in 30-50 ml of tetrahydrofuran, and add 0.57 g (5 mmol) of 30% hydrogen peroxide under stirring. Then, drop concentrated ammonia water to adjust the pH value of the solution to 6-7 to obtain A solution. Add 0.25mol / L ferrous chloride ethanol solution 20mL dropwise to solution A under stirring. Continue to stir and reflux for 2 to 3 hours. Suction filtration, washing with ethanol, drying to obtain the iron catalyst of general formula (II). The product yield is about 95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com