Method for preparing biological diesel oil from waste animals and plants grease
A technology of animal and vegetable oils and biodiesel, which is applied in the preparation of liquid hydrocarbon mixtures, biological raw materials, biofuels, etc., can solve the problems of high reaction temperature, difficulty in complete reaction, and poor effect of water removal, and achieve full esterification reaction , less equipment investment, faster speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
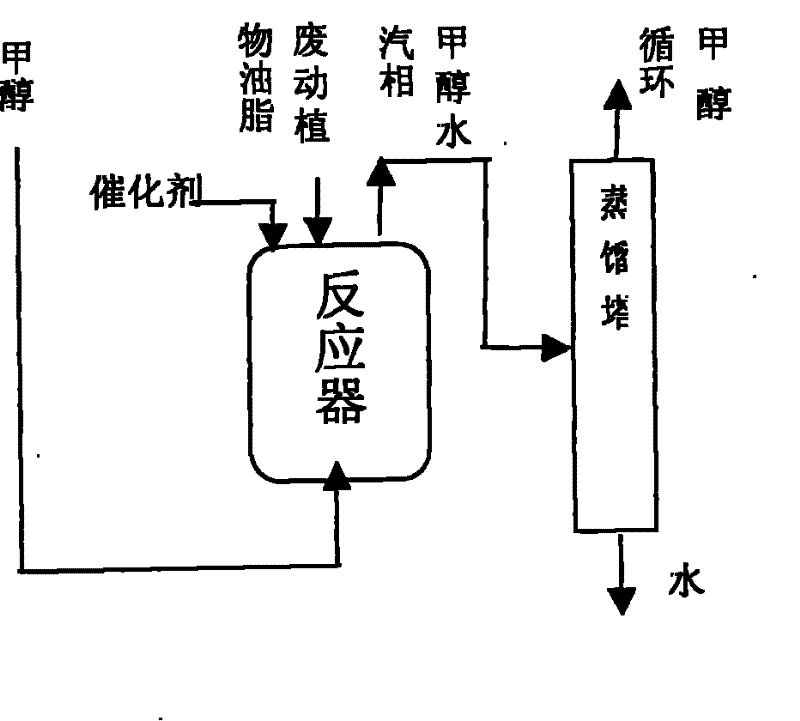
[0015] Example 1: In 3M 3 In the reactor, add 2000KG of acidified oil (the main component is fatty acid, acid value 189.3mgKOH / g oil, the oil content is very low and no transesterification reaction is necessary), stir and heat at about 65°C, add catalyst sulfuric acid 20KG, liquid phase methanol Continuously pumping from the bottom of the reactor, the temperature of the reactor rises to about 75°C to carry out the esterification reaction, the vapor phase (methanol, water) coming out from the top of the reactor directly enters the methanol rectification tower, and the liquid phase methanol after the water is separated, Return and add from the bottom of the reactor. When the acid value drops to 0.75mgKOH / g, the esterification reaction ends (about 2 hours), and the reaction solution settles for 4 hours (or directly centrifuged), and the sulfuric acid catalyst and a small amount of impurities in the lower layer are separated. , to obtain the upper layer crude biodiesel (acid value...
Embodiment 2
[0016] Example 2: In 3M 3 In the reactor, add clay oil (the main component is vegetable oil, a small amount of fatty acid, acid value 14.21mgKOH / g oil) 2000KG, stir and heat at about 65°C, add catalyst sulfuric acid 18KG, and continuously add liquid phase methanol from the bottom of the reactor to carry out esterification. For chemical reaction, the temperature of the reactor is gradually raised to about 75°C, and the vapor phase (methanol, water) coming out of the reactor directly enters the methanol rectification tower. After the reaction until the acid value dropped to 1mgKOH / g, the esterification reaction ended (about 0.5 hours), and the reaction solution settled for 4 hours to separate the lower layer of sulfuric acid catalyst and a small amount of impurities, and the upper layer was oil with an acid value of 0.92mgKOH / g.
[0017] Then, the clay oil after the esterification reaction is transesterified with methanol under the catalysis of the alkali catalyst, and settled f...
Embodiment 3
[0018] Example 3: In 3M 3 In the reactor, add 2000KG of refined waste oil (the main components are animal and vegetable oils, fatty acids, acid value 65.86mgKOH / g oil), and add solid catalyst Fe 2 o 3 / SO 4 2- / Carrier 40KG, stirring and heating at about 70°C, liquid phase methanol is continuously added from the bottom of the reactor for esterification reaction, the temperature of the reactor is gradually increased to about 100°C, and the vapor phase (methanol, water) coming out of the reactor is directly into the methanol distillation column. After reacting to the acid value and dropping to 2mgKOH / g, the esterification reaction ended (about 1.5 hours), and the reaction solution settled for 4 hours to separate the lower catalyst iron sulfate and a small amount of impurities, and the acid value of the upper layer reactant was 0.82mgKOH / g oil.
[0019] Then, the waste oil after the esterification reaction is transesterified with methanol under the catalysis of the alkali cat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com