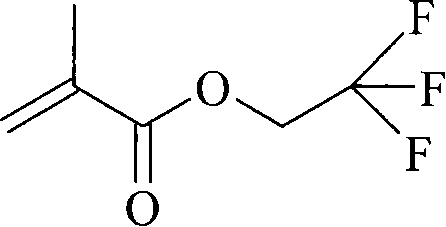
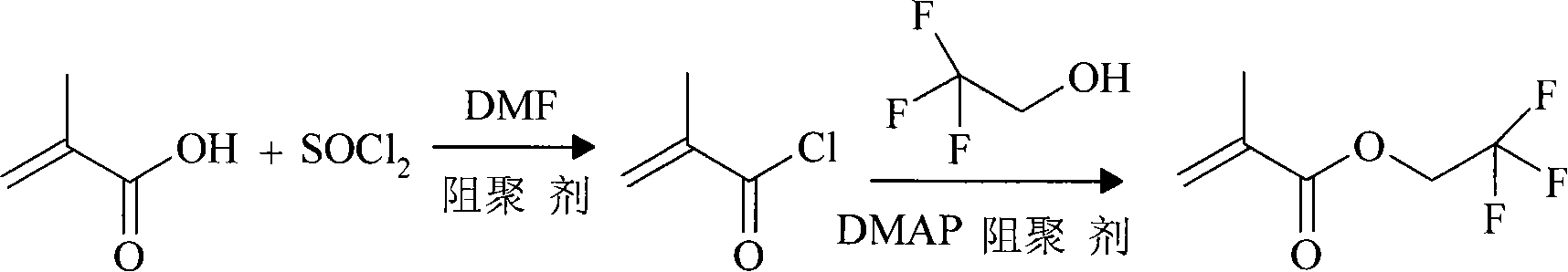
Method for preparing 2,2,2-trifluoroethyl methacrylate
A technology of methacrylic acid and trifluoroethyl ester, applied in the chemical field, can solve the problems of difficult storage and transportation of raw materials, harsh reaction system requirements, expensive raw materials, etc., and achieves the effects of high yield, low price and good purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Add 120g (1mol) of thionyl chloride and 0.8g of phenothiazine into a reactor equipped with a heater, a stirrer, a thermometer, and a reflux condenser, and slowly add methacrylic acid and DMF dropwise under stirring at 20-25°C. The mixed solution (prepared by mixing 93g of 1.1mol methacrylic acid and 1ml of DMF) was heated to 50±2°C and kept for 2 hours for reaction. Then slowly drop the mixed solution of 2,2,2-trifluoroethanol and 4-dimethylaminopyridine (DMAP) (90g 0.9mol 2,2,2-trifluoroethanol is mixed with 0.4g DMAP), control After reacting at 60±5°C for 4 hours, cool the reaction liquid to room temperature, and add 20wt% NaOH solution for neutralization, adjust the pH of the reaction liquid to 7, separate the organic layer, wash with saturated brine, add anhydrous MgSO 4 Dry, then distill under reduced pressure, collect the cut of 45-47 ℃ / 140mmHg, obtain 143.8g colorless transparent liquid product, be the product -2,2,2-trifluoroethyl methacrylate, the productive ra...
Embodiment 2
[0025] Add 120g (1mol) of thionyl chloride and 1g of hydroquinone into a reactor equipped with a heater, stirrer, thermometer, and reflux condenser, and add a mixture of methacrylic acid and DMF dropwise under stirring at 20-25°C solution (prepared by mixing 84.5g of 1.0mol methacrylic acid and 1ml of DMF), heated to 50±2°C and incubated for 3 hours. Then slowly drop the mixture of 2,2,2-trifluoroethanol and DMAP (80g 0.8mol2,2,2-trifluoroethanol mixed with 0.5g DMAP), and control the temperature at 60±5°C for 4.5 hours Afterwards, the reaction solution was cooled to room temperature, neutralized by adding 20wt% NaOH solution, and the pH of the reaction solution was adjusted to 7, and the organic layer was separated, washed with saturated brine, and added with anhydrous MgSO 4 Dry, then distill under reduced pressure, collect the cut of 45-47 ℃ / 140mm Hg, obtain 143.5g colorless transparent liquid product, be product methacrylic acid-2,2,2-trifluoroethyl ester, productive rate ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com