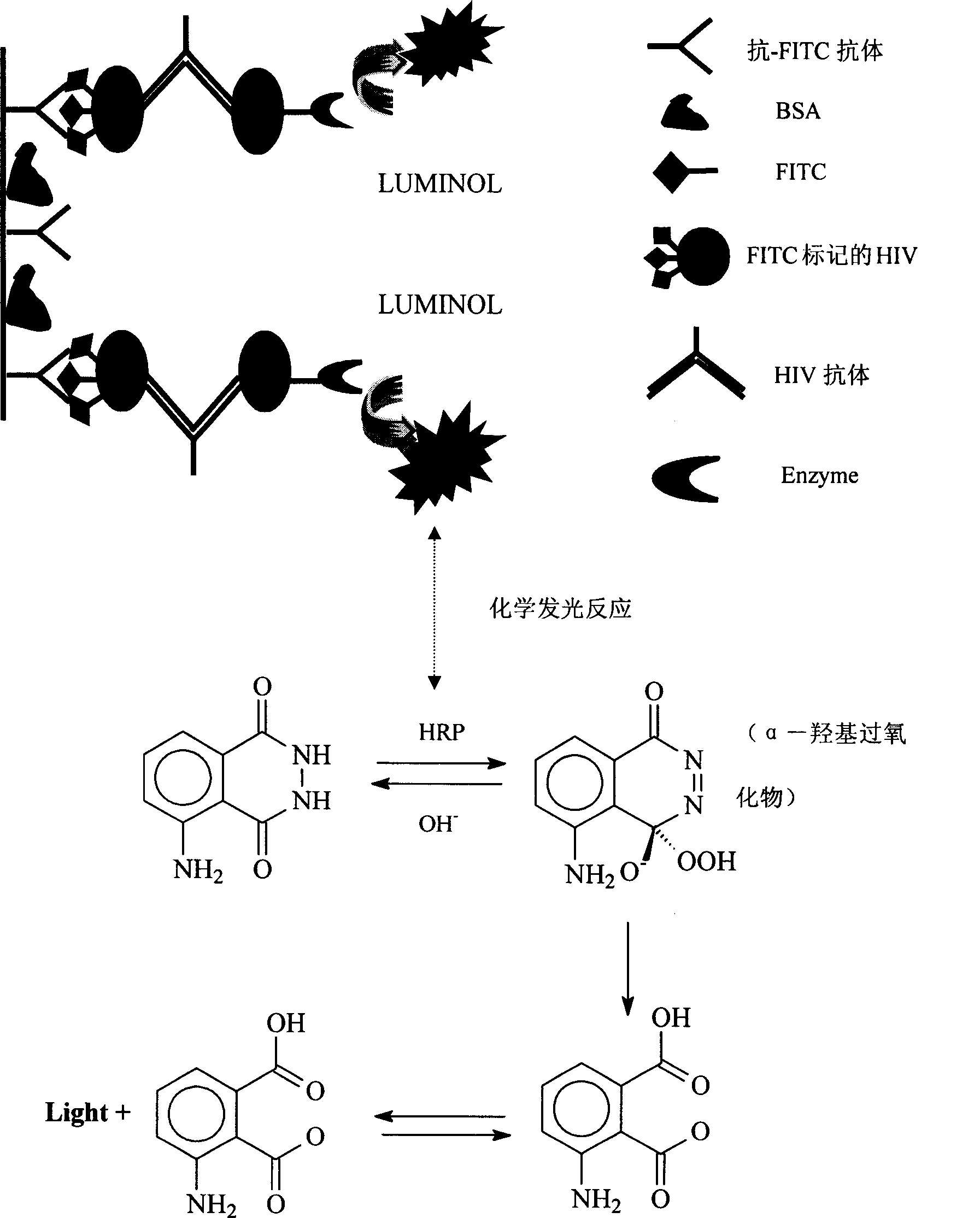
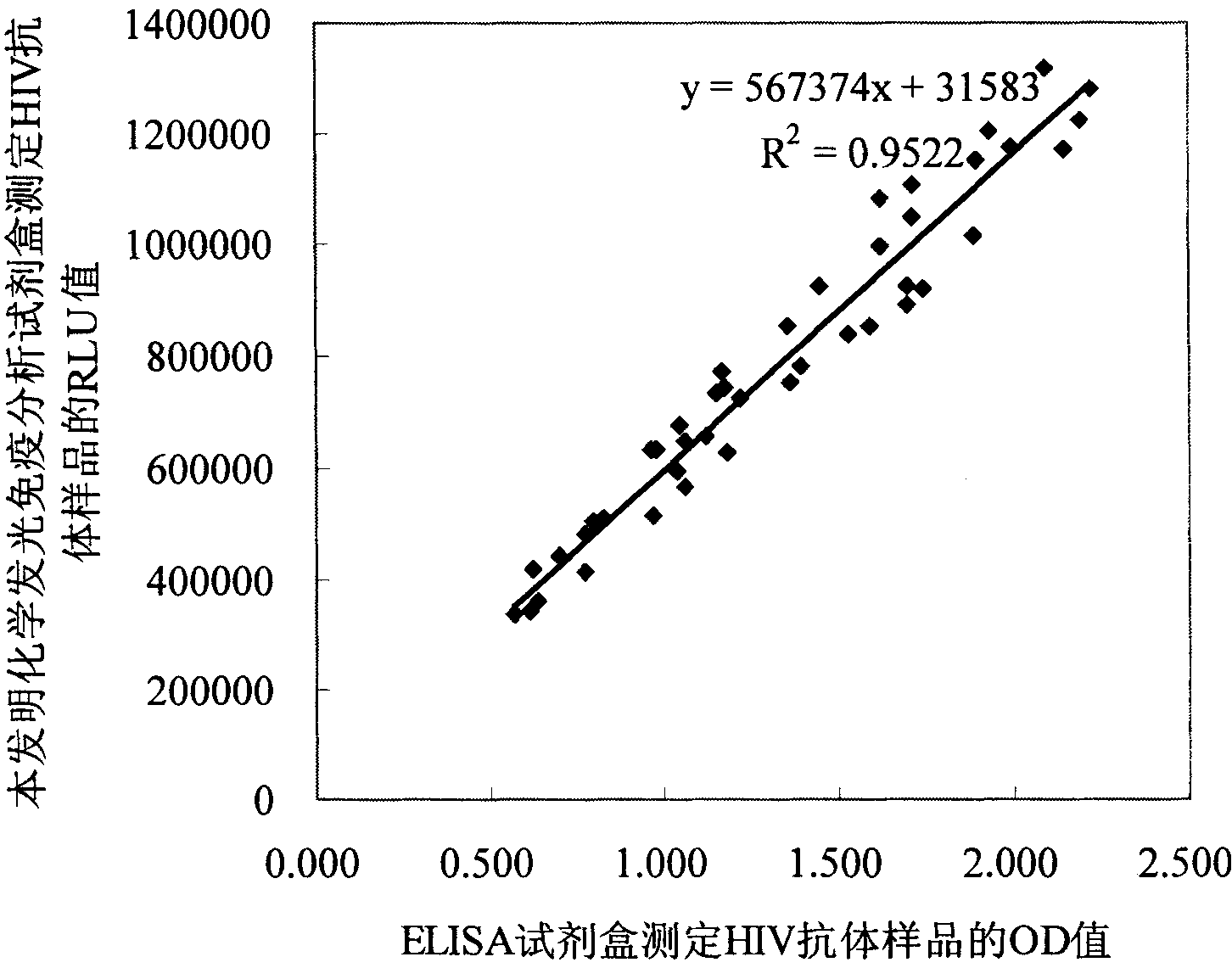
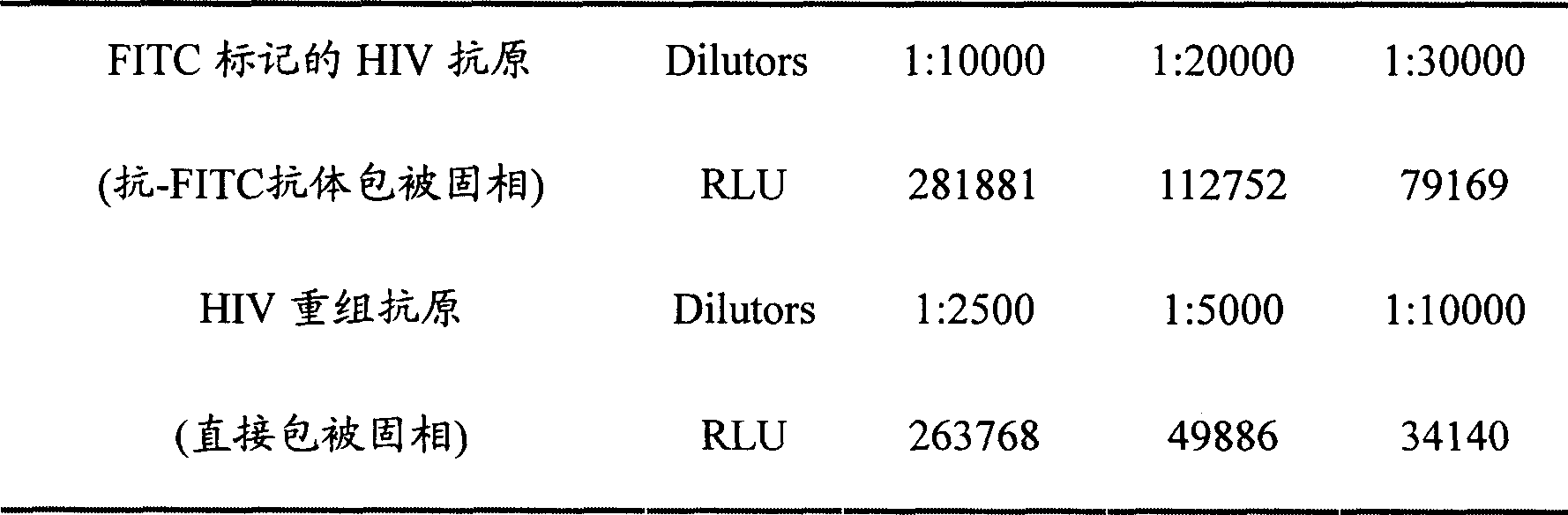
Human immunodeficiency virus antibody chemiluminescence immune analyzing diagnose reagent box and method of producing the same
A technology for human immunodeficiency and chemiluminescence immunity, which is applied in the field of immunoanalysis medicine, can solve the problems of uniformity and poor reproducibility of diagnostic kits, and achieve the effects of low cost, strong specificity and affinity, and improved sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1 prepares HIV antibody diagnostic kit of the present invention
[0035] HIV antibody diagnostic kit of the present invention comprises:
[0036] 1) 96-well or 48-well luminescent microplates pre-coated with anti-FITC antibody;
[0037] 2) FITC-labeled gp41, gp120, gp36 HIV recombinant antigens;
[0038] 3) HIV recombinant antigen labeled with horseradish peroxidase;
[0039] 4) The negative control solution is normal human serum;
[0040] 5) The positive control solution is a positive mixed slurry diluted with newborn bovine serum;
[0041] 6) The sample diluent is 0.1M Tris-HCl buffer, 0.1% BSA, and 0.1% Proclin300, pH7.4;
[0042] 7) The 20-fold concentrated washing solution is 2M Tris-HCl buffer solution containing 10% physiological saline, pH=8.5;
[0043] 8) The chemiluminescent substrates A and B are luminol solution, hydrogen peroxide and special effect enhancer (p-iodophenol) solution respectively.
[0044] Prepare the above kit by the following ...
Embodiment 2
[0067] Embodiment 2 prepares HIV antibody diagnostic kit of the present invention
[0068] Except that plastic beads are used as carrier, HIV recombinant antigen is labeled with alkaline phosphatase, AMPPD is used as chemiluminescent substrate, and the pH of the citrate buffer solution used in the coating process is 4.5, with the same method as in Example 1 Methods Prepare the kit of the present invention.
Embodiment 3
[0069] Embodiment 3 The using method of kit of the present invention:
[0070] 1. Take out the kit from the refrigerator at 4°C, equilibrate at room temperature for 20 minutes, take a bottle of concentrated lotion, add distilled water (1ml+19ml) for later use.
[0071] 2. Take the luminescent plate out of the sealed bag, add 100 μL FITC-labeled HIV recombinant antigen solution (1:2000) to the microtiter plate coated with anti-FITC antibody, and incubate at 37°C for 1 hour.
[0072] 3. Discard the liquid in each well, fill each well with the washing solution, let it stand for 10-20 seconds, shake off the washing solution; repeat the plate washing, and finally pat dry on clean absorbent paper.
[0073] 4. Set a blank control well without adding sample solution, set negative control 3 wells, and positive control 2 wells, take negative control solution, positive control solution and sample solution 50 μl each in the corresponding plate well, and then add 50 μl of enzyme marker , ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com