Selectivity M4 acceptor antagonist and medical use thereof
A technology of medicinal salts and toxicity, applied in the field of selective M4 receptor antagonists and their medical applications, can solve the problems that selective M4 receptor antagonists have not been found yet
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 12
[0022] Example 12'-cyclopentyl-2'-phenyl-2'-hydroxyacetic acid-9α-[N(2,2,2-trichloroethoxyformyl)-3-azabicyclo (3.3.1 ) nonyl] ester (III) synthesis
[0023] 10g (25mmol) of 2'-cyclopentyl-2'-phenyl-2'-hydroxyacetic acid-9α-[3-methyl-3-azabicyclo(3.3.1)nonyl]ester hydrochloride (I ) was added in 80mL of ether, and ammonium was added dropwise to make alkalization, so that I was converted into a free base and dissolved in ether, washed with water, dried and evaporated to remove ether under reduced pressure; 20mL of anhydrous benzene was added to the residue, and evaporated to dryness under reduced pressure; repeat three times. Finally, dissolve the free base in 30 mL of anhydrous benzene, add a solution of 8.3 g of 2,2,2-trichloroethoxycarbonyl chloride in 20 mL of benzene, and then add 300 mg of anhydrous potassium carbonate. Stir and heat for 5 hours, after cooling, filter off the solid, and evaporate the filtrate to dryness under reduced pressure. The residue was dissolved...
Embodiment 22
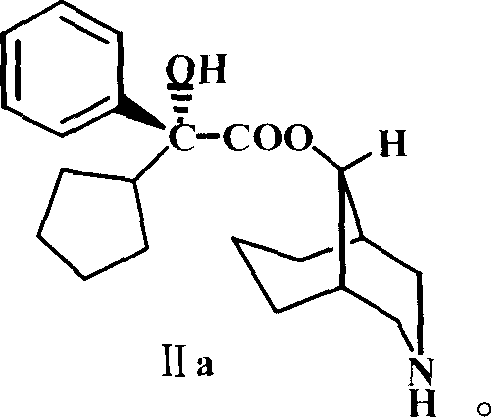
[0024] Example 2 Preparation of 2'-cyclopentyl-2'-phenyl-2'-hydroxyacetic acid-9-[3-azabicyclo(3.3.1)nonyl]ester (II)
[0025] Dissolve 11g (21mmol) III in 30mL of ethyl acetate, add 25mL of 90% acetic acid solution, add 7g of zinc powder several times under stirring, keep the temperature at 50°C; after adding the zinc powder, stir and heat in a water bath at 40°C 2 hours. Then add 90% 25mL acetic acid solution and 5g zinc powder. Stir and heat in a 50°C water bath for 12 hours. Silica gel thin layer detection, the reaction is basically complete. The solid was filtered off and washed with ethanol. The combined filtrates were evaporated to dryness under reduced pressure, 150 mL of ether was added, and alkalized with 5% sodium hydroxide solution to dissolve the free base in ether. The ether layer was separated, washed with water until neutral, dried over anhydrous magnesium sulfate, and the solvent was evaporated under reduced pressure to obtain a light yellow solid, which w...
Embodiment 3
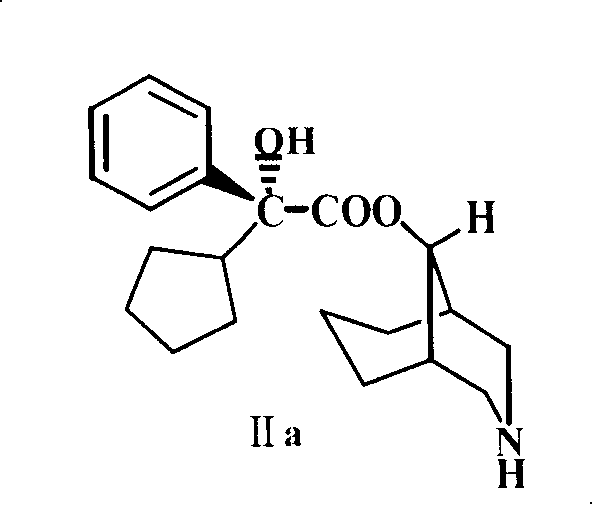
[0026] Example 3 (-)-2'-cyclopentyl-2'-phenyl-2'-hydroxyacetic acid-9-[3-azabicyclo(3.3.1)nonyl]ester (Levo-demethylphencyclononyl Preparation of Esters, IIa)
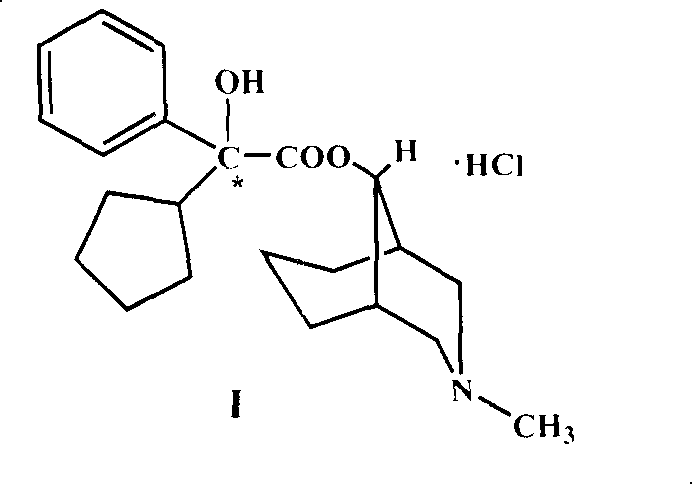
[0027]Dissolve 14.7g (0.1mol) of L-glutamic acid in 100mL of 2N sodium hydroxide, heat in a water bath at about 70°C, and add 22.8g (0.12mol) of p-toluenesulfonyl chloride in batches for about half an hour while stirring. The sodium hydroxide solution was continuously added dropwise to keep the pH ≥ 9, and the temperature was kept at 70° C. to react and stir for one hour. Cool to room temperature, cool in an ice-salt bath to below 0°C, and add concentrated hydrochloric acid dropwise to pH=3 or so. Extract three times with 300ml ethyl acetate, combine the extracts, heat to reflux, decolorize with activated carbon, and filter. Dry it with anhydrous calcium chloride, filter, and wash the desiccant with anhydrous ethyl acetate. Concentrate the solution to 80ml, and crystallize (L-(+)-N-p-toluenesulfonylglutamic acid 27....
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com