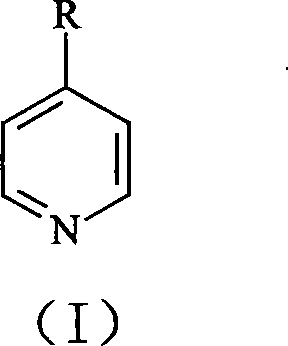
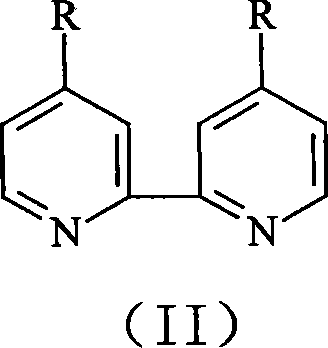
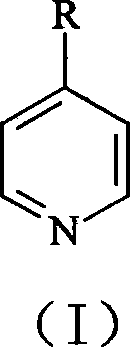
Synthesis of 4,4í»disubstituted-2,2í»-dipyridine
A synthetic method and a secondary substitution technology, applied in 4 fields, can solve problems such as energy waste, waste of energy, and increase production costs, and achieve the effects of simplifying equipment and operating procedures, reducing energy loss, and improving reaction rates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Synthesis of 4,4'-Dimethyl-2,2'-bipyridine
[0026] 4-methylpyridine 25.0g is mixed with 0.5g palladium / activated carbon catalyst (the palladium content of this catalyzer is 10wt.%), is packed in the stainless steel reaction kettle with polytetrafluoroethylene liner, with high-purity N 2 Bubble air for 15 minutes to remove dissolved oxygen therein, close the reactor, heat, and react at 180° C. for 15 hours while maintaining magnetic stirring; cool, filter the resulting mixture, and spin the filtrate to remove unreacted 4-picoline. 18.0 g of unreacted 4-methylpyridine was collected, and the white precipitate precipitated after rotary evaporation was recrystallized with ethyl acetate to obtain 5.2 g of 4,4'-dimethyl-2,2'-bipyridine, yield It was 74.3%, and the measured melting point was 175-179°C.
[0027] 1 H NMR (DMSO, 300MHz) δ: 2.41 (s, 6H, 2CH3), 7.27 (d, J=4.9Hz, 2H, H-5, H-5'), 8.23 (s, 2H, H-3, H -3'), 8.52 (d, J=4.9 Hz, 2H, H-6, H-6').
[0028] Elemental an...
Embodiment 2
[0030] Synthesis of 4,4'-Dimethyl-2,2'-bipyridine
[0031] The 4-picoline of 100.0g is mixed with 5 platinum / activated carbon catalysts (the platinum content of this catalyzer is 5wt.%), is packed in the stainless steel reactor with polytetrafluoroethylene liner, with N 2 Bubble air for 20 minutes, close the reactor, heat, and react at 200°C for 48 hours while maintaining magnetic stirring; cool, filter the resulting mixture, spin the filtrate to remove unreacted 4-methylpyridine, and collect 4-methylpyridine 10.5 g of 4,4'-dimethyl-2,2'-bipyridine was obtained by recrystallizing the white precipitate separated out after rotary evaporation with ethyl acetate, and the yield was 80.1%. It is 175-179°C.
[0032] 1 H NMR (DMSO, 300MHz) δ: 2.41(s, 6H, 2CH 3 ), 7.27(d, J=4.9Hz, 2H, H-5, H-5'), 8.23(s, 2H, H-3, H-3'), 8.52(d, J=4.9Hz, 2H, H-6, H-6').
Embodiment 3
[0034] Synthesis of 4,4'-diethyl-2,2'-bipyridine
[0035] The 4-ethylpyridine of 50.0g is mixed with 5.0g palladium / activated carbon catalyst (the palladium content of this catalyzer is 10wt.%), is charged in the stainless steel reaction kettle with polytetrafluoroethylene liner, with Ar gas bubbling 20 Minutes, remove dissolved oxygen therein, close the reactor, heat, and react at 200°C for 30 hours, during which magnetic stirring is maintained; after cooling, the resulting mixture is filtered to obtain a filtrate, and the unreacted 4-ethylpyridine is removed by rotary distillation of the filtrate, collected 41.7g of 4-ethylpyridine was obtained, and the white precipitate precipitated after rotary evaporation was recrystallized with ethyl acetate to obtain 6.5g of 4,4'-diethyl-2,2'-bipyridine with a yield of 78.3% .
[0036] Elemental analysis calculated value (C 14 h 16 N 2): C 79.25, H 7.55, N 13.21; Measured value: C 79.10, H 7.76, N 13.14 (%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com