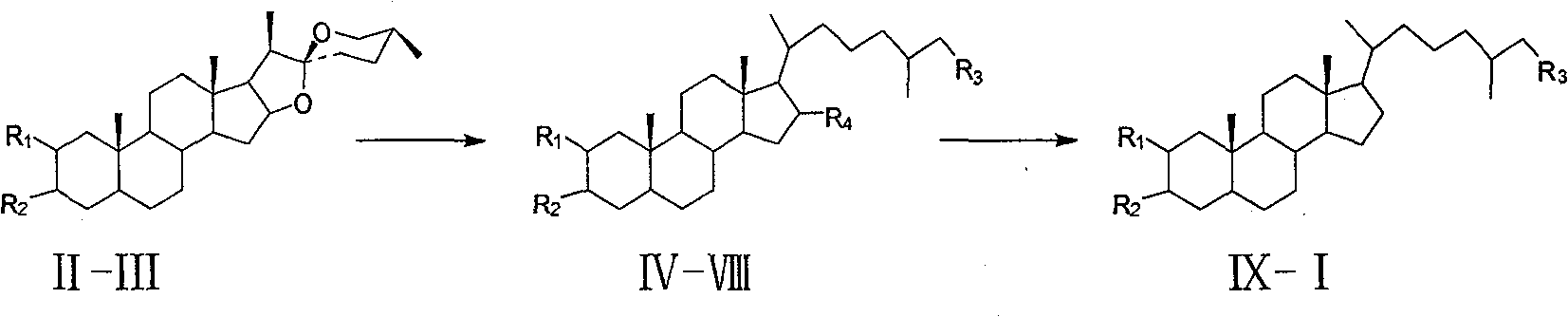
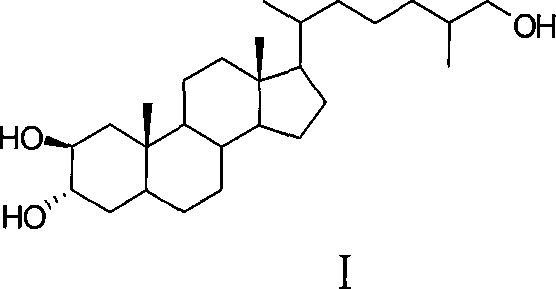
Synthesis of polyhydroxy ocean steroid (25R)-5 alpha-cholesteric-2 beta,3 alpha,26-triol
A synthetic method, -26-O- technology, applied in the direction of steroids, organic chemistry, etc., can solve the problem of low content and achieve a scientific and reasonable synthesis route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The synthetic method of (25R)-5α-cholesta-2β, 3α, 26-triol provided in Example 1 comprises the following steps:
[0025] (1). Dissolve 50 g (0.12 mol) of tegagenin II in 250 ml of dry pyridine, add 45.8 g (0.24 mol) of p-toluenesulfonyl chloride, and stir at room temperature for 24 hours. The reaction solution was slowly poured into 1000ml of 17% (w / w) HCl, a white precipitate was precipitated, filtered with suction, washed with water until neutral, and dried to obtain 68g of white solid compound III, with a yield of 99%.
[0026] (2). Compound III 30g (0.053mol) was dissolved in 450ml dry DMF, and LiBr 45.6g (0.53mol) and LiBr were added 2 CO 3 39.2g (0.53mol), refluxed for 1.5h. After cooling to room temperature, it was slowly poured into 10% (w / w) HCl, filtered with suction, washed with water until neutral, and dried to obtain 23g of compound IV as a white solid powder. Yield 87.8%.
[0027] (3). Add compound IV (15g, 37.7mmol) and 1100ml ethanol successively in ...
Embodiment 2
[0034] The synthetic method of (25R)-5α-cholesta-2β, 3α, 26-triol provided in Example 2 comprises the following steps:
[0035] (1). With embodiment 1. (1).
[0036] (2). Compound III 30g (0.053mol) was dissolved in 450ml dry DMF, and LiBr 45.6g (0.53mol) and LiBr were added 2 CO 3 39.2g (0.53mol), refluxed at 110°C for 4h. After cooling to room temperature, it was slowly poured into 10% (w / w) HCl, filtered with suction, washed with water until neutral, and dried to obtain 23g of compound IV as a white solid powder with a yield of 82%.
[0037] (3)~(8). Same as embodiment 1 (3)~(8).
Embodiment 3
[0039] The synthesis method of (25R)-5α-cholesta-2β, 3α, 26-triol provided in Example 3 comprises the following steps:
[0040] (1)~(2). With embodiment 1.(1)~(2).
[0041] (3). Add compound IV (15g, 37.7mmol) and 1100ml ethanol successively in a 2L three-neck round bottom flask equipped with mechanical stirring, constant pressure dropping funnel, and reflux condenser, add 445g of zinc amalgam, and drip under reflux Concentrated hydrochloric acid 300ml was added, the reaction was tracked by TLC, and the reaction was stopped after the disappearance of the raw material. Filter, remove most of the ethanol under reduced pressure, extract 250ml x 3 times with dichloromethane, wash the organic layer with water until neutral, dry over anhydrous sodium sulfate, and concentrate to obtain 13g of white solid. Purification by silica gel column chromatography (ethyl acetate:petroleum ether, V:V=3:7) gave 10.8 g of compound V as a white solid, with a yield of 65%.
[0042] (4)~(8). With e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com