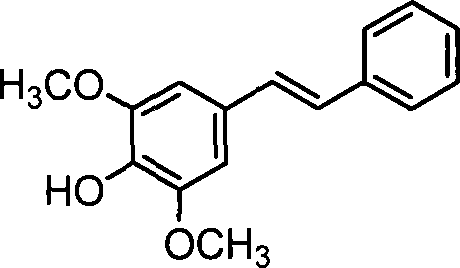
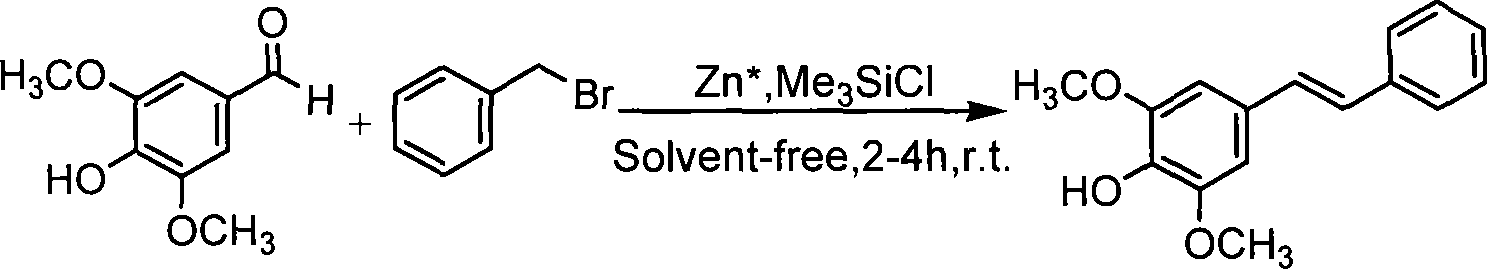Compound (E)-4-hydroxy-3,5-dimethoxystilbene and preparation thereof
A technology of dimethoxystilbene and dimethoxybenzaldehyde, which is applied in the field of polyhydroxystilbene compound-4-hydroxy-3, can solve the problems of poor stereoselectivity, low yield, and many reaction steps, and achieve the synthesis The effect of short route, high efficiency and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] (1) Activation of zinc powder: wash off the oxide film on the surface of zinc powder with 2 to 5% dilute hydrochloric acid, wash the zinc powder with water, acetone, ether, and ethanol in sequence, remove inorganic salts and moisture in the zinc powder, vacuum dry, Cool to obtain activated zinc powder;
[0040] (2) Preparation of (E)-4-hydroxyl-3,5-dimethoxystilbene: 4-hydroxyl-3,5-dimethoxybenzaldehyde and activated zinc powder are added in a molar ratio of 1:1.15 In a dry reactor, under the protection of nitrogen, gradually add 4-hydroxy-3,5-dimethoxybenzaldehyde 2 times the molar amount of trimethyl chloride to the reactor at a rate of 3-5ml / min. Silane and 1.1 times the molar amount of benzyl bromide were reacted under stirring for 2 hours. After the reaction was completed by TLC, 1 times the molar amount of 4-hydroxy-3,5-dimethoxybenzaldehyde was added successively. Ethyl ether and 2 times The molar amount of saturated ammonium chloride solution was stirred, and t...
Embodiment 2
[0042] (1) Activation of zinc powder: same as embodiment one.
[0043] (2) Preparation of (E)-4-hydroxyl-3,5-dimethoxystilbene:
[0044] Add 4-hydroxyl-3,5-dimethoxybenzaldehyde and activated zinc powder into the dry reactor at a molar ratio of 1:1.2, and feed into the reactor at 3-5ml / min under the protection of nitrogen. Gradually add 2.25 times the molar amount of 4-hydroxy-3,5-dimethoxybenzaldehyde trimethylchlorosilane and 1.2 times the molar amount of benzyl bromide, react under stirring for 4 hours, TLC tracking until the reaction is complete Finally, add 4-hydroxy-3,5-dimethoxybenzaldehyde 1.5 times the molar amount of ether and 2.5 times the molar amount of saturated ammonium chloride solution, stir, separate the organic phase, extract the aqueous phase with ether, and combine into the organic phase, and dried with anhydrous magnesium sulfate, and separated by column chromatography (silica gel, 300-400; petroleum ether: ethyl acetate, 10:1) after evaporating the solv...
Embodiment 3
[0046] (1) Activation of zinc powder: same as embodiment one.
[0047] (2) Preparation of (E)-4-hydroxyl-3,5-dimethoxystilbene:
[0048] Add 4-hydroxyl-3,5-dimethoxybenzaldehyde and activated zinc powder into the dry reactor at a molar ratio of 1:1.3, and feed into the reactor at 3-5ml / min under the protection of nitrogen. 4-Hydroxy-3, 5-dimethoxybenzaldehyde 1.8 times the molar amount of trimethylchlorosilane and 1.3 times the molar amount of benzyl bromide were gradually added, and reacted for 3 hours under stirring, and TLC tracked until the reaction was complete Finally, 4-hydroxy-3,5-dimethoxybenzaldehyde 1.0 times the molar amount of ether and 2.5 times the molar amount of saturated ammonium chloride solution were added successively, stirred, the organic phase was separated, the aqueous phase was extracted with ether, and combined into the organic phase, and dried with anhydrous magnesium sulfate, and separated by column chromatography (silica gel, 300-400; petroleum et...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com