Polycaprolactone/amylose amphiphilic block polymer as well as preparation method and use thereof
A technology of polycaprolactone and amylose, applied in the field of polycaprolactone/amylose amphiphilic block polymer and its preparation, can solve toxic side effects, non-biodegradability, and lack of reaction of molecular chains To achieve the effect of strong function, good biodegradability and biocompatibility, and abundant sources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
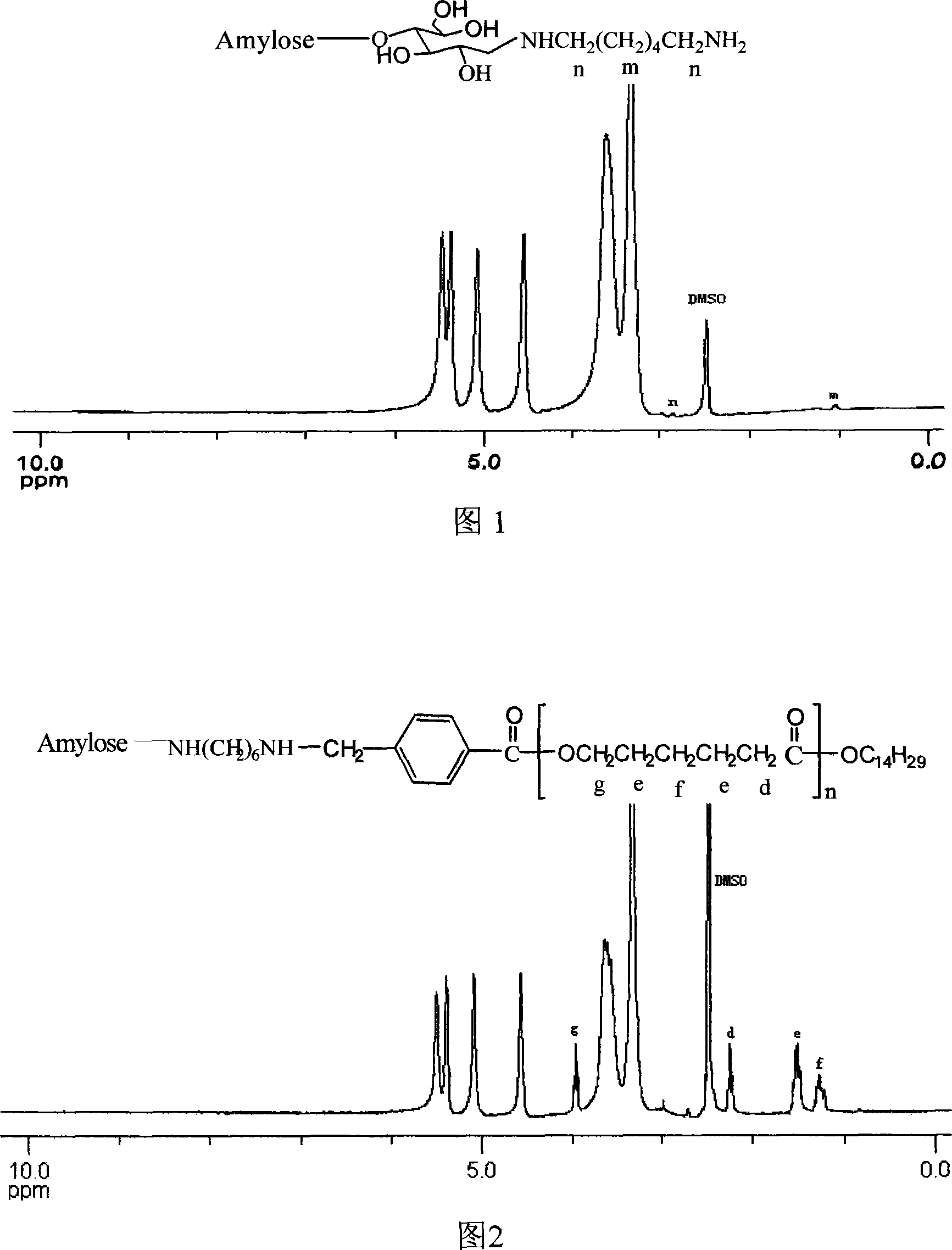
[0034] (1) Weigh myristyl alcohol, Sn(Oct) according to the ratio of substances 1:0.0055:55 2 , ε-CL in a polymerization tube, after repeated vacuuming / argon filling for 3 times, heat-seal the tube under vacuum state, and then place the polymerization tube in an oil bath at 130°C for 7 hours to react. After the reaction, dissolve with tetrahydrofuran to obtain a polymer solution, and use ether (10 times the volume of tetrahydrofuran) to precipitate the product hydroxyl-terminated polycaprolactone PCL-OH, which is filtered and dried under reduced pressure for future use. Yield: 90%.1 HNMR (in d 6 -DMSO, 300MHz) δppm: 1.40, 1.66, 2.33, 4.07 (CH 2 ); 0.89 (CH 3 ); 3.7 (CH 2 OH). through 1 HNMR analysis shows that the number average molecular weight of PCL-OH is 5914.
[0035] Dissolve 0.2mmol of PCL-OH and 0.4mmol of p-aldehyde benzoic acid in 20ml of tetrahydrofuran, add 10ml of tetrahydrofuran solution dissolved with 0.4mmol of DCC and 20mg of DMAP under stirring, and the...
Embodiment 2
[0039] (1) Weigh myristyl alcohol, Sn(Oct) according to the ratio of substances 1:0.008:80 2 , ε-caprolactone in a polymer tube, after repeated vacuuming / argon filling for 3 times, heat-seal the tube under vacuum, and then place the polymer tube in an oil bath at 130°C for 7 hours to react. After the reaction, the polymer solution was obtained by dissolving in tetrahydrofuran, and the product PCL-OH was precipitated with diethyl ether (11 times the volume of tetrahydrofuran), filtered, and dried under reduced pressure for future use. Yield: 91%. 1 HNMR (in d 6 -DMSO, 300MHz) δppm: 1.40, 1.66, 2.33, 4.07 (CH 2 ); 0.89 (CH 3 ); 3.7 (CH 2 OH). through 1 HNMR analysis showed that the number average molecular weight of PCL-OH was 8764.
[0040] Dissolve 0.2mmol of PCL-OH and 0.4mmol of p-aldehyde benzoic acid in 20ml of tetrahydrofuran, add 10ml of tetrahydrofuran solution dissolved with 0.4mmol of DCC and 20mg of DMAP under stirring, and then seal the reaction at room tempe...
Embodiment 3
[0044] (1) Weigh myristyl alcohol, Sn(Oct) according to the ratio of substances 1:0.009:45 2 , ε-caprolactone (ε-CL) in a polymerization tube, after repeated vacuuming / argon filling for 3 times, heat-seal the tube in a vacuum state, and then place the polymerization tube in an oil bath at 120°C React for 8 hours. After the reaction, the polymer solution was obtained by dissolving in tetrahydrofuran, and the product PCL-OH was precipitated with diethyl ether (12 times the volume of tetrahydrofuran), filtered, and dried under reduced pressure for future use. The number average molecular weight of the obtained PCL-OH was 5014.
[0045] Dissolve 0.2mmol of PCL-OH and 0.3mmol of p-aldehyde benzoic acid in 25ml of tetrahydrofuran, add 10ml of tetrahydrofuran solution dissolved with 0.3mmol of DCC and 14.6mg of DMAP under stirring, and then seal the reaction at room temperature for 1 day. After the reaction was completed, the resulting dicyclohexyl urea (DCU) precipitate was filter...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com