Method for preparing benzoxazine intermediate containing propargyl ether and resin thereof
A technology of propargyl ether and benzoxazine, which is applied in the field of thermosetting resin and its preparation, to achieve the effects of low toxicity, high heat resistance and good thermal performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
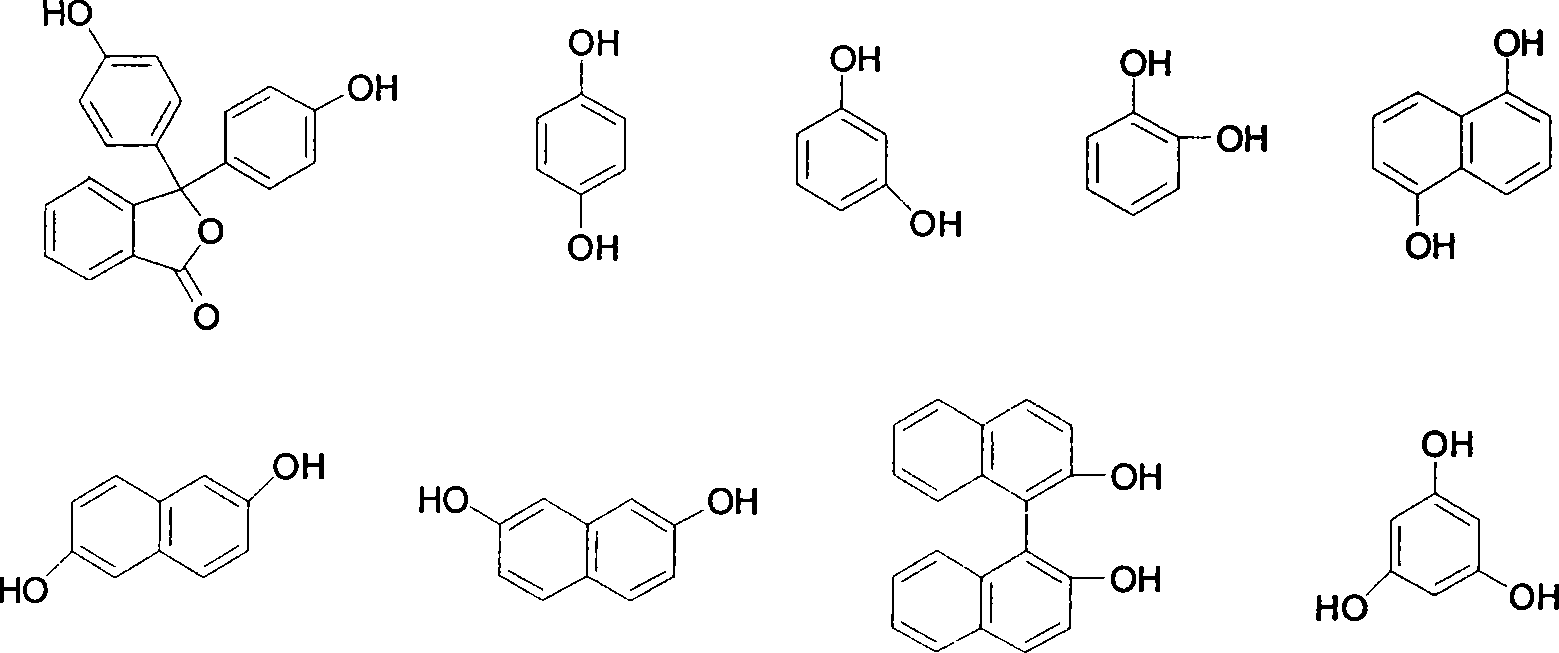
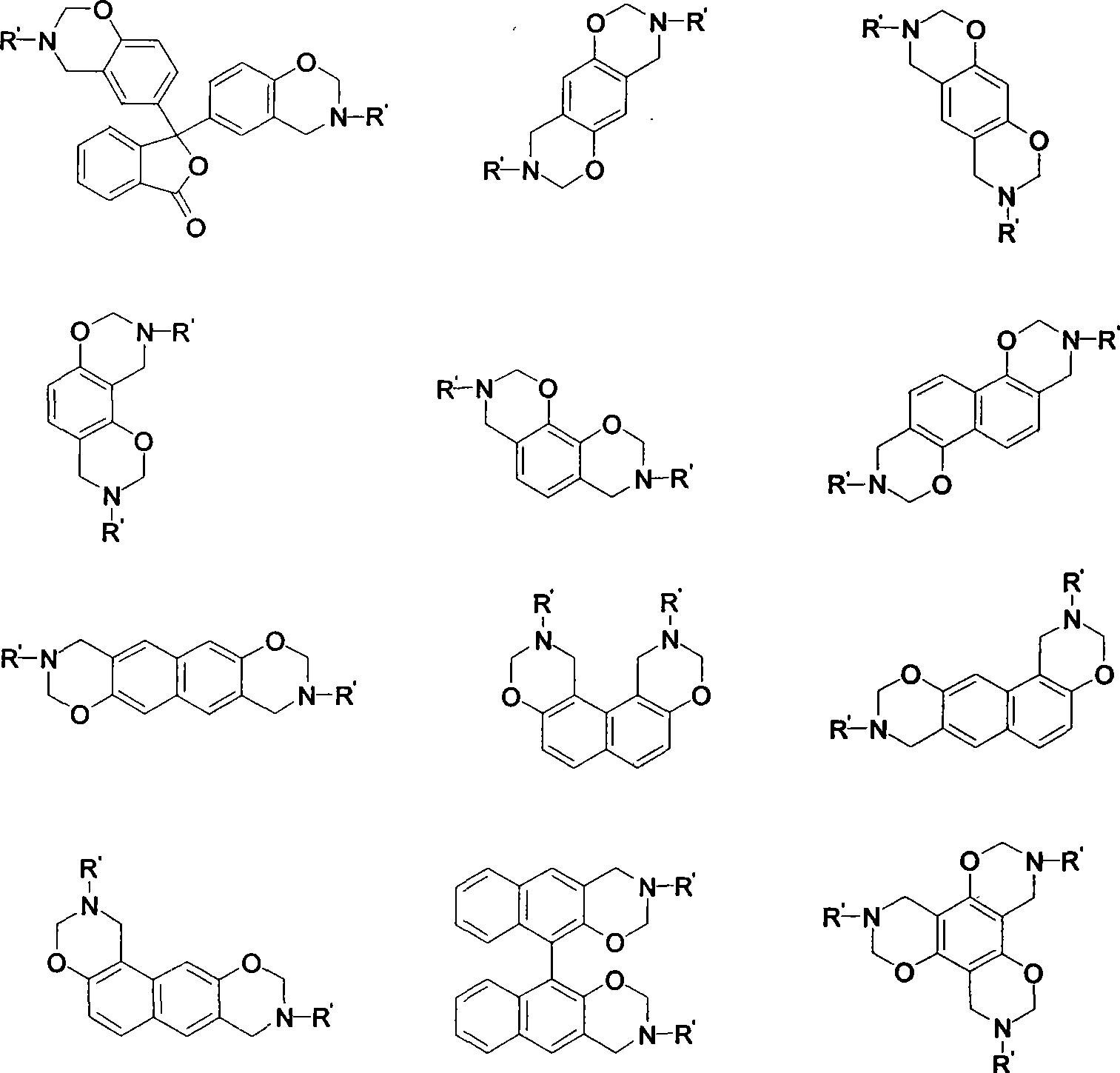
[0038] 3,3'-bis(3-(4-propargyl ether)phenyl-3,4-dihydro-2H-1,3-benzoxazine)-1(3H)-isobenzofuranone The synthesis of p-aminophenyl propargyl ether, phenolphthalein and formaldehyde is the preparation process of raw material synthesis benzoxazine resin:
[0039] Add 100ml of dioxane, 0.7g of triethylamine and 19ml of 37% formaldehyde solution in sequence into a 250ml three-neck flask equipped with a stirrer and a condenser, and stir to mix. Slowly add 14.7g p-aminophenyl propargyl ether, control the reaction temperature not to exceed 30°C, and adjust the pH value to 7 with triethylamine to generate N-dimethylol compound and keep it stable in the solution; react for 15 minutes Then add 16g of phenolphthalein, raise the temperature to reflux, and stop the reaction after reacting the mixed solution at the reflux temperature for 5 hours, so that the N-dimethylol compound and the phenolic hydroxyl group and its ortho-position on the benzene ring will undergo condensation reaction, de...
Embodiment 2
[0043] 3,3'-bis(3-(2-propargyl ether)phenyl-3,4-dihydro-2H-1,3-benzoxazine)-1(3H)-isobenzofuranone The synthesis of o-aminophenyl propargyl ether, phenolphthalein and formaldehyde is the preparation process of raw material synthesis benzoxazine resin:
[0044] Add 90ml of dioxane, 0.84g of triethylamine and 21ml of 36% formaldehyde solution in sequence into a 250ml three-neck flask equipped with a stirrer and a condenser, and stir to mix. Slowly add 16.5g o-aminophenyl propargyl ether, control the reaction temperature not to exceed 30°C, and adjust the pH value to 7 with triethylamine to generate N-dimethylol compound and keep it stable in the solution; react for 15 minutes Then add 18g of phenolphthalein, raise the temperature to reflux, and stop the reaction after reacting the mixed solution at the reflux temperature for 5 hours, so that the N-dimethylol compound and the phenolic hydroxyl group and its ortho position on the benzene ring will undergo condensation reaction, an...
Embodiment 3
[0048] 3,3'-bis(3-(3-propargyl ether)phenyl-3,4-dihydro-2H-1,3-benzoxazine)-1(3H)-isobenzofuranone The synthesis of, take m-aminophenyl propargyl ether, phenolphthalein and formaldehyde as the preparation technology of raw material synthetic benzoxazine resin:
[0049] Add 110ml of dioxane, 0.7g of triethylamine and 16ml of 35% formaldehyde solution in sequence into a 250ml three-neck flask equipped with a stirrer and a condenser, and stir to mix. Slowly add 14.7g m-aminophenyl propargyl ether, control the reaction temperature not to exceed 30°C, and adjust the pH value to 7 with triethylamine to generate N-dimethylol compound and keep it stable in the solution; react for 15 minutes Then add 14g of phenolphthalein, raise the temperature to reflux, and stop the reaction after reacting the mixed solution at the reflux temperature for 5 hours, so that the N-dimethylol compound and the phenolic hydroxyl group and its ortho position on the benzene ring will undergo condensation rea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com