A tin nickel alloy negative material of lithium ion battery and its making method
A tin-nickel alloy negative electrode and lithium-ion battery technology, applied in electrode manufacturing, battery electrodes, electrolytic coatings, etc., can solve poor electrochemical stability, poor compatibility between metal powder and binder, and easy powdering and falling off of electrode active materials and other problems, to achieve the effect of easy operation, elimination of residual mechanical stress, and prevention of agglomeration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
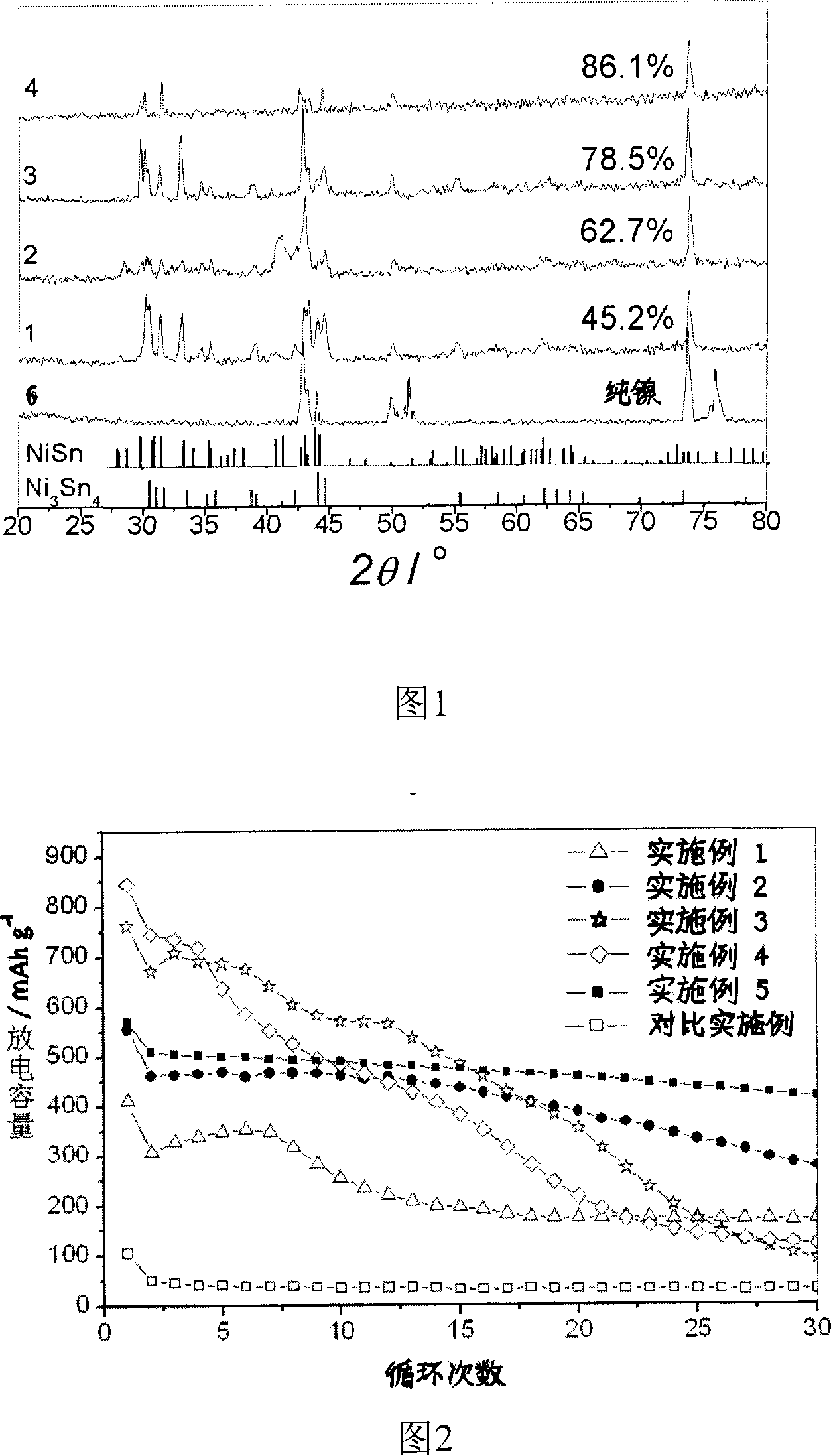
Image
Examples
Embodiment 1
[0025] (1) 0.9mol K 4 P 2 o 7 ·3H 2 O and 0.1mol glycine were dissolved in a certain amount of deionized water, and then 0.10mol SnCl 2H 2 O, 0.35mol NiCl 2 ·6H 2 Add O to the above solution to dissolve, add water to make up to 1L, and finally add NH 4 OH to adjust the pH to 7.5.
[0026] (2) Add the electroplating solution prepared in the step (1) into the electroplating tank, connect the tin sheet and the nickel sheet together as the anode, and use the negative electrode current collector copper foil (thickness is 10 microns) as the cathode for the lithium ion battery , one side of the copper foil is coated with oil-based pen and ink, and one side is covered to achieve single-sided electroplating. At 45°C, the current density is 0.05A·dm -2 Under the condition of electroplating for 10 minutes;
[0027] (3) Wash off the ink on the electroplated copper foil in acetone, wash it three times with distilled water, dry it at room temperature, and dry it in vacuum at 100°C f...
Embodiment 2
[0031] (1) 0.9molK 4 P 2 o 7 ·3H 2 O and 0.1mol glycine were dissolved in a certain amount of deionized water, and then 0.15mol SnCl·2H 2 O, 0.30mol NiCl 2 ·6H 2 Add O to the above solution to dissolve, add water to make up to 1L, and finally add NH 4 OH to adjust the pH to 8.
[0032] (2) Add the electroplating solution prepared in the step (1) into the electroplating tank, connect the tin sheet and the nickel sheet together as the anode, and use the negative electrode current collector copper foil (10 microns in thickness) as the cathode for the lithium ion battery , one side of the copper foil is coated with oil-based pen and ink, and one side is covered to achieve single-sided electroplating. At 40°C, the current density is 0.1A·am -2 Under the condition of electroplating for 10 minutes;
[0033] (3) Wash the electroplated copper foil in acetone to remove the ink, then wash it twice with distilled water, dry it at room temperature, and dry it in vacuum at 100°C for...
Embodiment 3
[0037] (1) 0.9mol K 4 P 2 o 7 ·3H 2 O and 0.1mol glycine were dissolved in a certain amount of deionized water, and then 0.23mol SnCl·2H 2 O, 0.22mol NiCl 2 ·6H 2 Add O to the above solution to dissolve, add water to make up to 1L, and finally add NH 4 OH to adjust the pH to 8.5.
[0038] (2) Add the electroplating solution prepared in the step (1) into the electroplating tank, connect the tin sheet and the nickel sheet together as the anode, and use the negative electrode current collector copper foil (thickness is 10 microns) as the cathode for the lithium ion battery , one side of the copper foil is coated with oil-based pen and ink, and one side is covered to achieve single-sided electroplating. At 30°C, the current density is 0.15A·dm -2 Electroplating for 5 minutes under the condition;
[0039] (3) Wash the electroplated copper foil in acetone to remove the ink, then wash it twice with distilled water, dry it at room temperature, and dry it in vacuum at 100°C for...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com