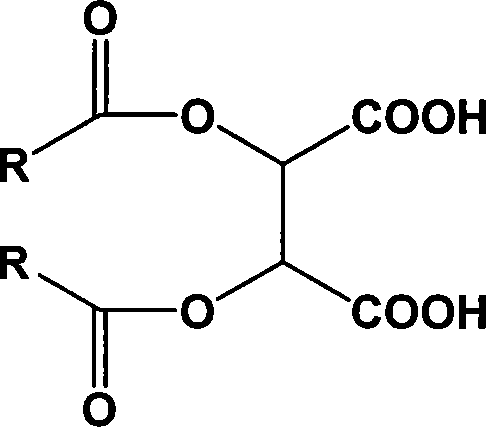
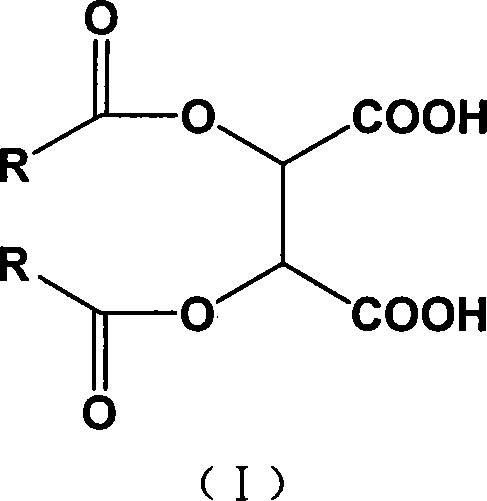
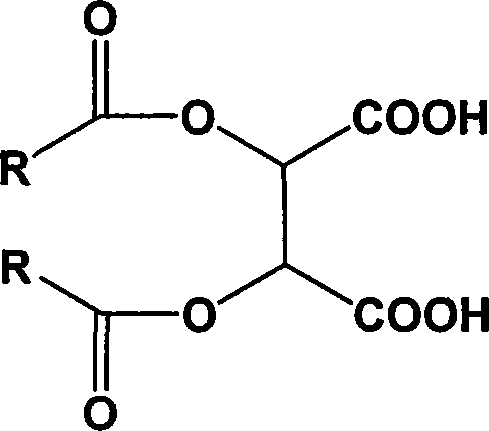
Chiral tartaric acids derivatives and preparation method thereof
A technology for tartaric acid and derivatives, which is applied in the field of derivatization preparation by utilizing chiral tartaric acid hydroxyl groups, can solve the problems of high toxicity and corrosiveness of trichlorotoluene, difficult industrialized production, and limited applicability, and achieves good ecological benefits, preparation The effect of low cost and various structures
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0024] The preparation method of the above-mentioned chiral tartaric acid derivatives comprises the following steps: (1) in a solvent environment, the tartaric acid and the acid chloride are catalyzed, and after the acid chloride is added dropwise, thionyl chloride is added to the reaction system to promote the reaction to be carried out thoroughly (2) hydrolyzing the acid anhydride product obtained in step (1) to obtain chiral tartaric acid derivatives of the above formula; (3) post-processing the reaction waste liquid and recovering acid chlorides by means of acid-base intermodulation.
[0025] In the step (1), in a solvent environment, add a catalyst to the tartaric acid, and add 2-2.6 times the acid chloride of the tartaric acid dropwise to the reaction system between 90°C and 100°C, calculated by moles; Calculated in terms of moles, after the dropwise addition of the acid chloride, add 0.2-1 times the amount of thionyl chloride to the reaction system dropwise, and keep sti...
Embodiment 1
[0029] Embodiment 1: the preparation of L-dibenzoyl tartaric acid
[0030] 1) Preparation of L-dibenzoyl tartaric anhydride
[0031]Add 300ml of toluene and 100g of L-tartaric acid into a 1000ml reaction vessel (with condenser tube, tail gas absorption, thermometer, dropping funnel), stir and add a catalytic amount of zinc chloride. Stir and heat to between 90°C and 95°C, and add dropwise a toluene solution containing 216g of benzoyl chloride. Drop it at 90°C--95°C within about 3 hours. Weigh 96g of thionyl chloride and add it dropwise between 90°C and 95°C for about 3 hours. Slowly raise the temperature to 105°C--110°C, and keep the temperature constant for 2 hours. After natural stirring and cooling for 4 hours, cool down to normal temperature with cooling water. The reaction system was pumped under reduced pressure to remove excess hydrogen chloride gas. After filtration, the filter cake was soaked and washed with a small amount of ethyl acetate, dried at 60° C. for 12...
Embodiment 2
[0034] Embodiment 2: the resolution effect of L-dibenzoyl tartaric acid
[0035] Put 1000g of DL-aspartic acid-β-methyl ester in a 1000mL three-neck flask, add 200mL of water to dissolve all DL-aspartic acid-β-methyl ester, raise the temperature to 90°C, add 124g of L-DBTA, The solid dissolved immediately, and then cooled to room temperature, a large number of crystals precipitated, filtered, and dried to obtain 154 g of D-aspartic acid·L-DBTA salt, with a yield of 88.5%. Melting point: 150-152°C [α] D 20 (Methanol) = -60.4°.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com