2-methoxy estradiol vena NANO emulsions
A methoxyestradiol and nanoemulsion technology, applied in the field of medicine, can solve the problems of easily causing hemolysis and allergic reactions, poor clinical treatment compliance, lack of sustained release and targeting, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
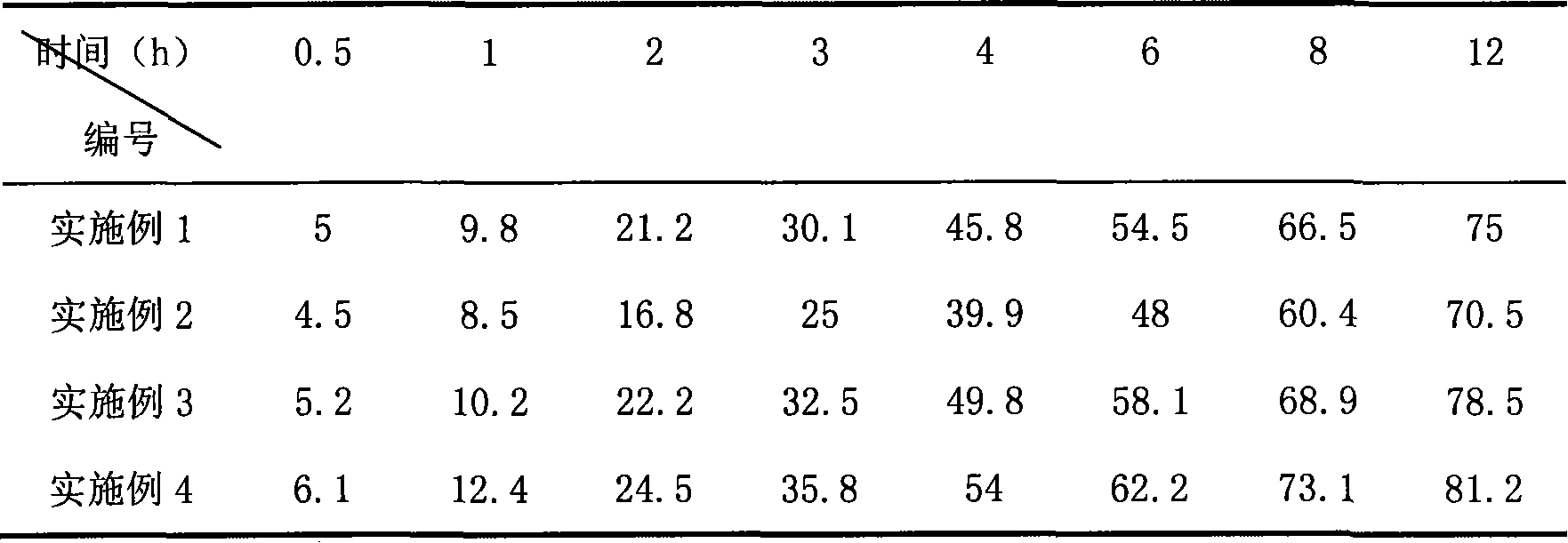
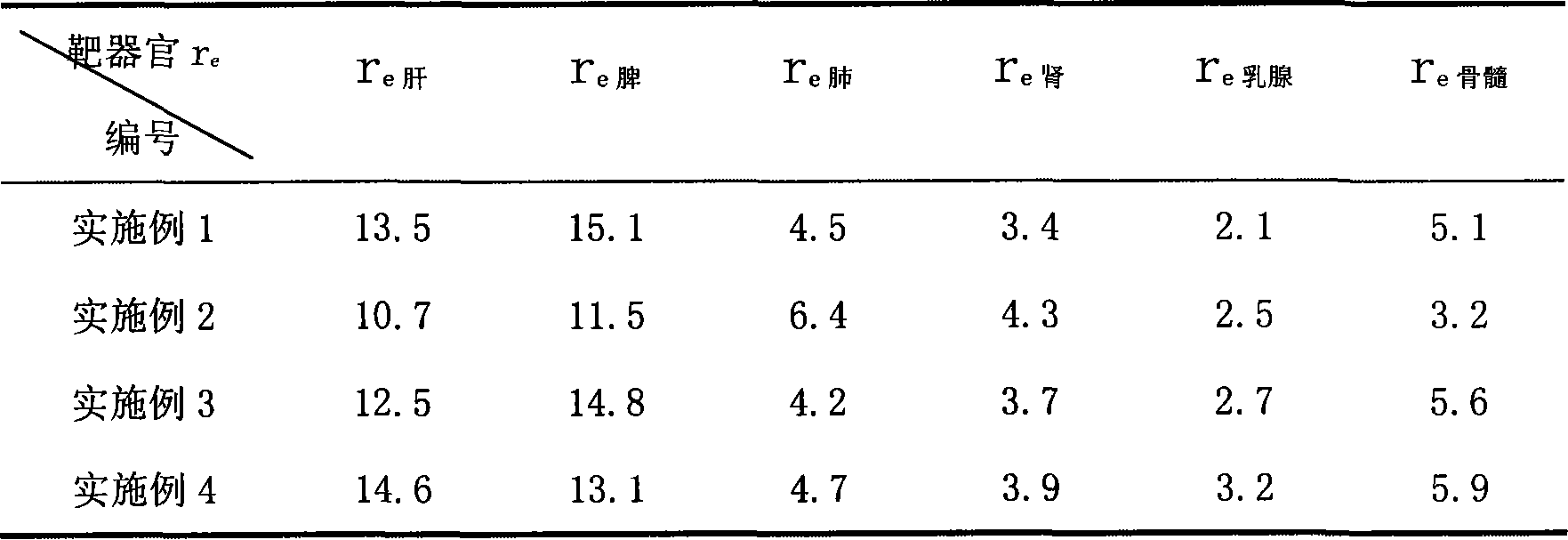
[0020] Weigh 200 mg of 2-methoxyestradiol and 2.0 g of soybean lecithin, dissolve them in 5 ml of chloroform, evaporate to dryness under reduced pressure with a rotary thin-film evaporator, and remove the chloroform to form a drug liquid crystal film. 20g of soybean oil, shake to disperse the liquid crystal film, add 4% glucose solution filtered through a 0.22μm microporous membrane to 100ml, stir to form colostrum, and then go through the micro-jet high-pressure milk homogenizer three times, the operating pressure is 17k psi , use 0.1Mol / L hydrochloric acid or 0.1Mol / L sodium hydroxide to adjust the pH value to 5.5-8.0, filter through a 0.45μm microporous membrane, purify nitrogen flow protection, fill and package, and sterilize with a rotary autoclave at 121°C After 20 minutes, rinse with hot water and gradually cool to room temperature.
[0021] The product of this embodiment, the average particle diameter 240nm, Zeta potential-35mV is measured by the laser scattering parti...
Embodiment 2
[0023]Weigh 150 mg of 2-methoxyestradiol and 1880.9 g of poloxamer, dissolve in 5 ml of chloroform, evaporate to dryness under reduced pressure with a rotary thin-film evaporation to remove the chloroform to form 2-methoxyestradiol and poloxamer Then add 20 g of olive oil filtered through a 0.22 μm microporous membrane, shake to disperse the solid dispersion, add 2.5% glycerin solution filtered through a 0.22 μm microporous membrane to 100 ml, stir to form colostrum, Then go through the micro-jet high-pressure milk homogenizer for three times, the operating pressure is 15k psi, adjust the pH to 5.5-8.0 with 0.1Mol / L hydrochloric acid or 0.1Mol / L sodium hydroxide, filter with a 0.45μm microporous membrane, and protect with purified nitrogen flow Fill and seal, rotate the autoclave at 121°C, sterilize for 20 minutes, pour hot water and gradually cool to room temperature, ready to serve.
[0024] The product of this embodiment, the average particle diameter 290nm, Zeta potential-...
Embodiment 3
[0026] Weigh 250 mg of 2-methoxyestradiol and 2.0 g of egg yolk lecithin, dissolve them in 5 ml of chloroform, evaporate to dryness under reduced pressure with a rotary thin-film evaporation to remove the chloroform to form a drug liquid crystal film, and then add in 0.22 μm microporous membrane filter 30g of soybean oil, shake to disperse the liquid crystal film, add the solution containing 0.5%% poloxamer and 5% sorbitol filtered through a 0.22μm microporous membrane to 100ml, stir to form colostrum, and then pass through the microjet The high-pressure milk homogenizer is homogenized three times, the operating pressure is 16k psi, the pH is adjusted to 5.5-8.0 with 0.1Mol / L hydrochloric acid or 0.1Mol / L sodium hydroxide, the 0.45μm microporous membrane is filtered, the nitrogen flow protection is filled and packaged, and the rotation Autoclave at 121°C, sterilize for 20 minutes, rinse with hot water and gradually cool to room temperature to get ready.
[0027] The product of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com