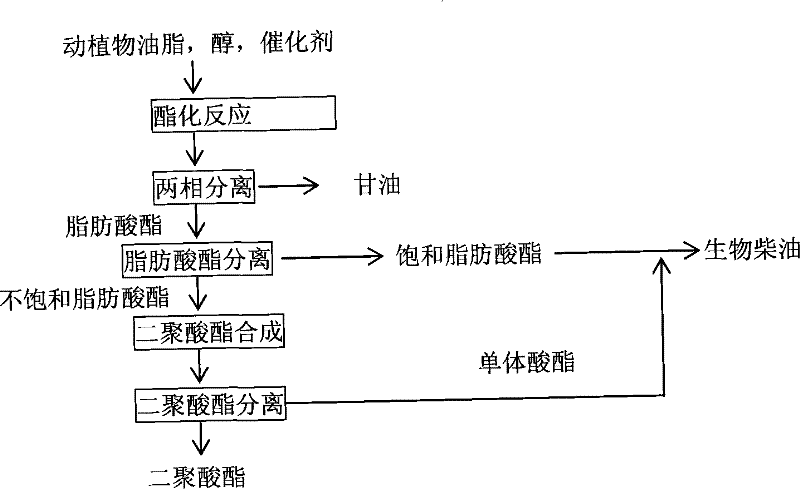
Method for coproducing biological diesel oil and dimeric dibasic acid ester by using animals and plants grease
A technology of animal and vegetable oils and biodiesel, which is applied in the direction of fatty acid esterification, oligomerization reaction to prepare carboxylic acid esters, biofuels, etc. It can solve the problems of reducing the concentration of reactants, increasing production constraints, and slowing down the polymerization reaction speed. To achieve the effect of increasing the polymerization reaction speed, shortening the reaction time and improving the quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] In a 500mL four-neck flask, add 300 grams of dried cottonseed oil and 31 grams of methanol (the molar ratio of oleyl alcohol to 1:3), stir and heat to reach the methanol reflux temperature of about 65°C, add 3 grams of solid alkali CaO, The reaction was refluxed for 3 hours. After the reaction was over, excess methanol was distilled off. The reaction solution was filtered while hot to separate the catalyst, and then the filtrate was poured into a separatory funnel and allowed to stand overnight to separate layers. Separate the lower floor by-product glycerol to obtain 288 grams of fatty acid methyl esters. The content of fatty acid methyl ester detected by chromatography is 96.5%.
[0024] Add 461 grams of 95% ethanol in 173 grams of urea, heat to reflux, after the urea dissolves completely, add 288 grams of fatty acid methyl esters (mixed fatty acid ester: urea: solvent mass ratio (W / W / W) that is preheated to 50 ℃ ) was 1:0.6:1.6), refluxed for 50min, cooled to 10°C...
Embodiment 2
[0027] In a 500mL three-necked flask equipped with a vacuum dehydration device, a thermometer, and a reflux condenser, add 300 grams of rapeseed oil, heat to 120°C, dehydrate under reduced pressure for 1-2 hours, cool to 50°C, add 150 grams of ethanol (oleyl alcohol) Molar ratio is 1: 10), 2.5 grams of potassium hydroxide, after reflux reaction for 5 hours, finish reaction, distill excess ethanol, be cooled to 50 ℃, then pour into the separatory funnel and stand for layering, separate the lower floor by-product glycerin , to obtain 298 grams of fatty acid ethyl ester. The fatty acid ethyl ester content detected by chromatography was 95.4%.
[0028] In 1192 grams of urea, add 2384 grams of methyl alcohol, heat to reflux, after the urea is completely dissolved, add 298 grams of fatty acid ethyl esters preheated to 50 ° C (mixed fatty acid ester: urea: solvent mass ratio (W / W / W) is 1:4:8), refluxed for 50min, cooled to 15°C, and stood still for 20 hours, a large amount of crysta...
Embodiment 3
[0031] In a 500mL three-necked flask equipped with a vacuum dehydration device, a thermometer and a reflux condenser, add 300 grams of waste edible oil, heat to 120°C, dehydrate under reduced pressure for 1-2 hours, cool to 50°C, add 83.5 grams of methanol (oleyl alcohol The molar ratio is 1: 8), 2.5 grams of 98% concentrated sulfuric acid, after reflux reaction for 5 hours, finish the reaction, distill off excess methanol, cool to 50°C, then pour into the separatory funnel and let stand for layering, separate the lower floor by-product Glycerol, to obtain 290 grams of fatty acid methyl esters. The content of fatty acid methyl ester detected by chromatography is 94.4%.
[0032] Add 1160 grams of methanol in 290 grams of urea, heat to reflux, after the urea is completely dissolved, add 290 grams of fatty acid methyl esters preheated to 50°C (mixed fatty acid ester: urea: solvent mass ratio (W / W / W) is 1:1:4), refluxed for 50min, cooled to 15°C, and stood still for 20 hours, a l...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com