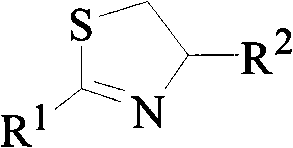
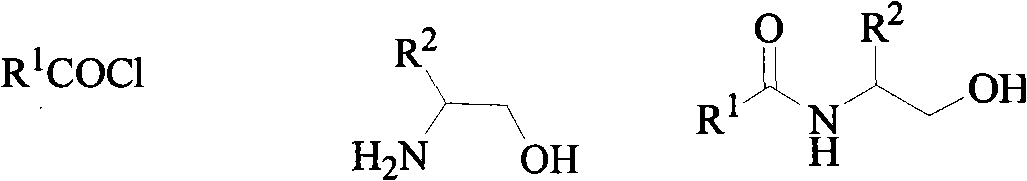
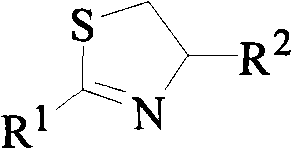
2,4-disubstituted thiazolines derivatives, preparing method and application thereof
A compound and thienyl technology, which is applied to 2,4-disubstituted thiazoline derivatives and the fields of their preparation and application, can solve the problem that the action level and duration of action are not completely satisfactory and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0029] The synthesis of example 1,2-furyl-4-methyl-thiazoline (CAU-FB-1)
[0030] Into a 50 mL round bottom flask was added 1.12 g (10 mmol) furan-2-carboxylic acid and 2 mL SOCl 2 , refluxed for 4 hours, and the excess SOCl was evaporated under reduced pressure with a rotary evaporator 2 , without further purification to give furan-2-yl chloride, which was then added with 10 mL of CH 2 Cl 2 stand-by. Add 50 mL of dichloromethane and 4 mL of triethylamine (Et 3 N) and 0.72g (10mmol) alaninol, cooled to 0°C in an ice bath, added dropwise the dichloromethane solution of the above-mentioned furan-2-acyl chloride within 30min under continuous rapid stirring, stirred at room temperature for 4 hours after dropping, and removed by rotary evaporator. solvent to obtain N-(1-methyl-2-hydroxyl) ethyl-2-furanamide; then add 50mL toluene, 5mL triethylamine (Et 3 N) and 3.33g (15mmol) of phosphorus pentasulfide, refluxed for 6 hours, cooled, poured out the supernatant, washed twice wit...
Embodiment 2
[0039] Embodiment 2, the preparation of compound CAU-FB series emulsifiable concentrate preparation
[0040] Add compound CAU-FB-1110mg in the 100mL volumetric flask, use the emulsifier (the emulsifier is polyoxyalkylene alkyl aryl ether) with mass percentage composition to be 12%, the mass percentage content to be 1% penetrant (penetrant The agent is dissolved in xylene solution of alkyl aryl sulfonate), and then the above-mentioned xylene solution is used to dilute to obtain the emulsifiable concentrate preparation containing compound CAU-FB-110.01%.
Embodiment 3
[0041] The antifungal activity determination of embodiment 3, CAU-FB series compound
[0042] Experimental method: In vitro growth rate assay was used.
[0043] Bacteriostatic solution: In the experiment, each compound of CAU-FB 1-15 was prepared into a 5000mg / L bacteriostatic solution with acetone.
[0044] Strains:
[0045]Rhizoctonia solani kühn, Phytophthora parasitica Dast, Sclerotinia sclerotiorum (Lib) de Bary, Pythium melon should be: (Pythiumaphanidermatum), Fulvia fulva (Cooke) Cif.), Fusarium oxysporum Schl.f.sp. Vasinfectum (Atk). Snyd & Hans..), Pyricularia oryzae Cav., Phomopsis asparagi (sacc .) Bubak), Macrophoma kawatsukaiHara.
[0046] Preparation of fungal block: Transfer the above-mentioned fungi to a PSA plate under aseptic conditions, place Sclerotinia sclerotiorum in a 23-25°C incubator, and cultivate the rest of the plant pathogenic fungi in a 27-28°C incubator until the hyphae are uniform Overgrown the PSA plate, and then made a bacterial block wit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com