Oral preparation containing cefa-iskia, preparation method and application thereof
A cephalexin and preparation technology, applied in the field of therapeutic drugs and preparation thereof, can solve the problems of low blood drug concentration, slow absorption, long time to reach effective blood drug concentration, etc., and achieves simple production process, fast onset of action, and improved dissolution rate. degree of effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1 - Cephalexin Candy
[0044] Prescription: cephalexin 125g, sucrose 818.5g, citric acid 10g, food coloring-400.1g, mannitol 10g, hypromellose 36.5g, preparation amount 1000 tablets.
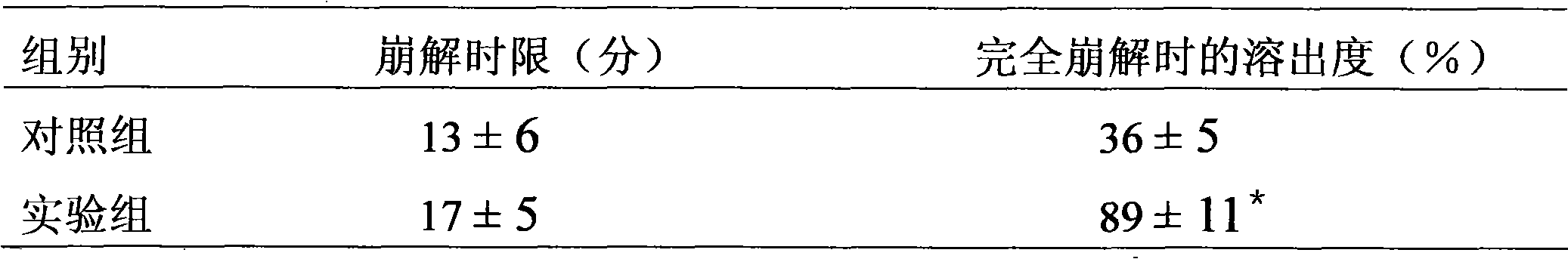
[0045] Method: Prepare a mixture of cephalexin 125g, citric acid 10g, food coloring-400.1g, mannitol 10g, and hypromellose 36.5g, grind it, and pass it through a 20-mesh sieve. Take 818.5 grams of sucrose, heat to 140°C to melt, cool to 85°C, add the sieved mixture, stir rapidly for 10 minutes, place on a product forming machine and extrude, each lozenge weighs 2 grams. Then measure the disintegration time limit of cephalexin candy in 37 ℃ 900ml water and the dissolution rate when disintegrating completely.
[0046] Results: The disintegration time limit was 28-35 minutes, and the dissolution rate was 84%-89% when completely disintegrated.
Embodiment 2
[0047] Example 2-low sugar cephalexin candy
[0048] Prescription: cephalexin 125g, sucrose 168.5g, fructooligosaccharide 300g, xylitol 300g, inulin 50g, citric acid 10g, food coloring-400.1g, mannitol 10g, hypromellose 36.5g, dosage 1000 tablets .
[0049] Method: Prepare a mixture of citric acid 10g, food coloring-400.1g, mannitol 10g, and hypromellose 36.5g, grind it, and pass it through a 20-mesh sieve. Take 168.5g of sucrose, 300g of fructooligosaccharides, 300g of xylitol, and 50g of inulin, mix them, heat to 140°C to melt, add the sieved mixture, stir rapidly for 10 minutes, cool to 85°C, add 125g of cephalexin, and quickly Stir for 10 minutes, place on a product forming machine and extrude, each lozenge weighs 2 grams. Then measure the disintegration time limit of cephalexin candy in 37 ℃ 900ml water and the dissolution rate when disintegrating completely.
[0050] Results: The disintegration time limit was 28-36 minutes, and the dissolution rate was 85%-91% when co...
Embodiment 3
[0051] Embodiment 3-cephalexin buccal tablet
[0052] Prescription: cephalexin 125 grams, hypromellose 128 grams, polyethylene glycol 6000 746 grams, 1 gram of aspartame.
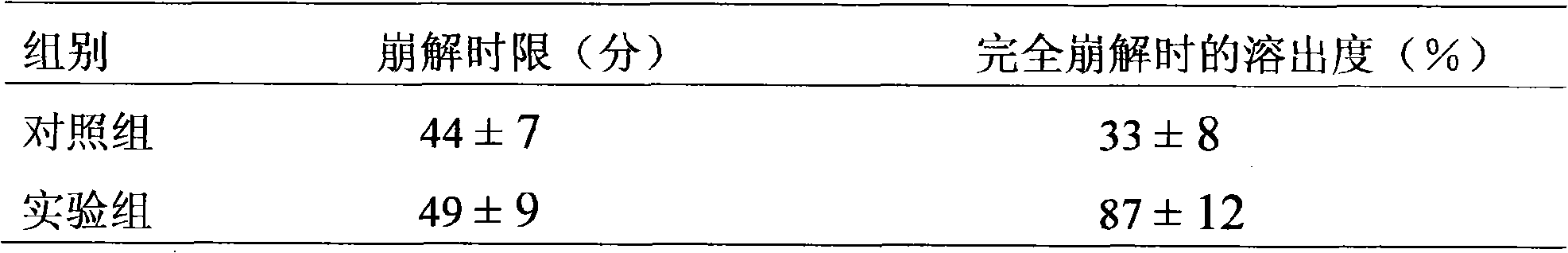
[0053] Method: Prepare 128 grams of hypromellose, polyethylene glycol 6000 746 grams, 1 gram of aspartame mixed matrix, heating and melting, cooling to 80 ° C, adding 125 grams of cephalexin, stirring and mixing, cooling at room temperature until solidified, granulating, and extruding on a tablet machine. Tablets weigh 1 gram. Then measure the disintegration time limit of cephalexin buccal tablet in 37 ℃ 900ml water and the dissolution rate when disintegrating completely.
[0054] Results: The disintegration time limit was 24-31 minutes, and the dissolution rate was 85%-92% when completely disintegrated.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com