Eugenol aspirin ester pharmaceutical compound, preparation and preparing method
A technology of eugenol aspirin ester and aspirin, applied in the field of eugenol aspirin ester medicinal compound and its formulation and preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
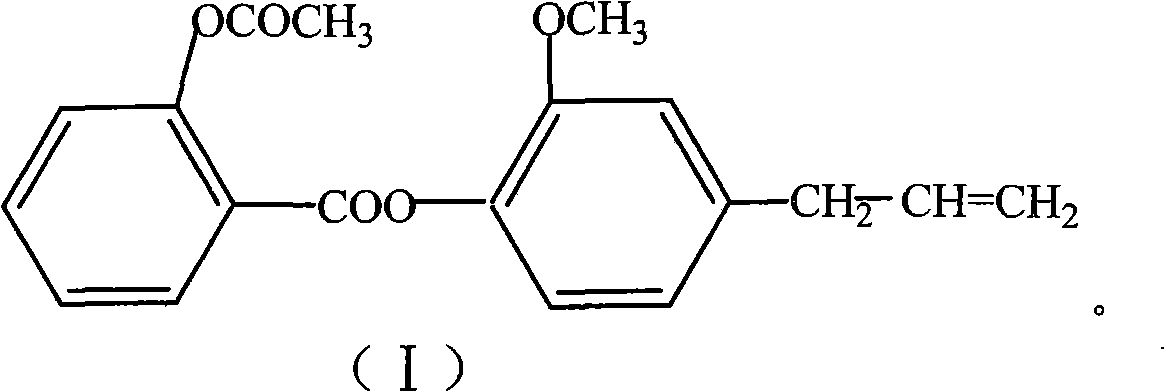
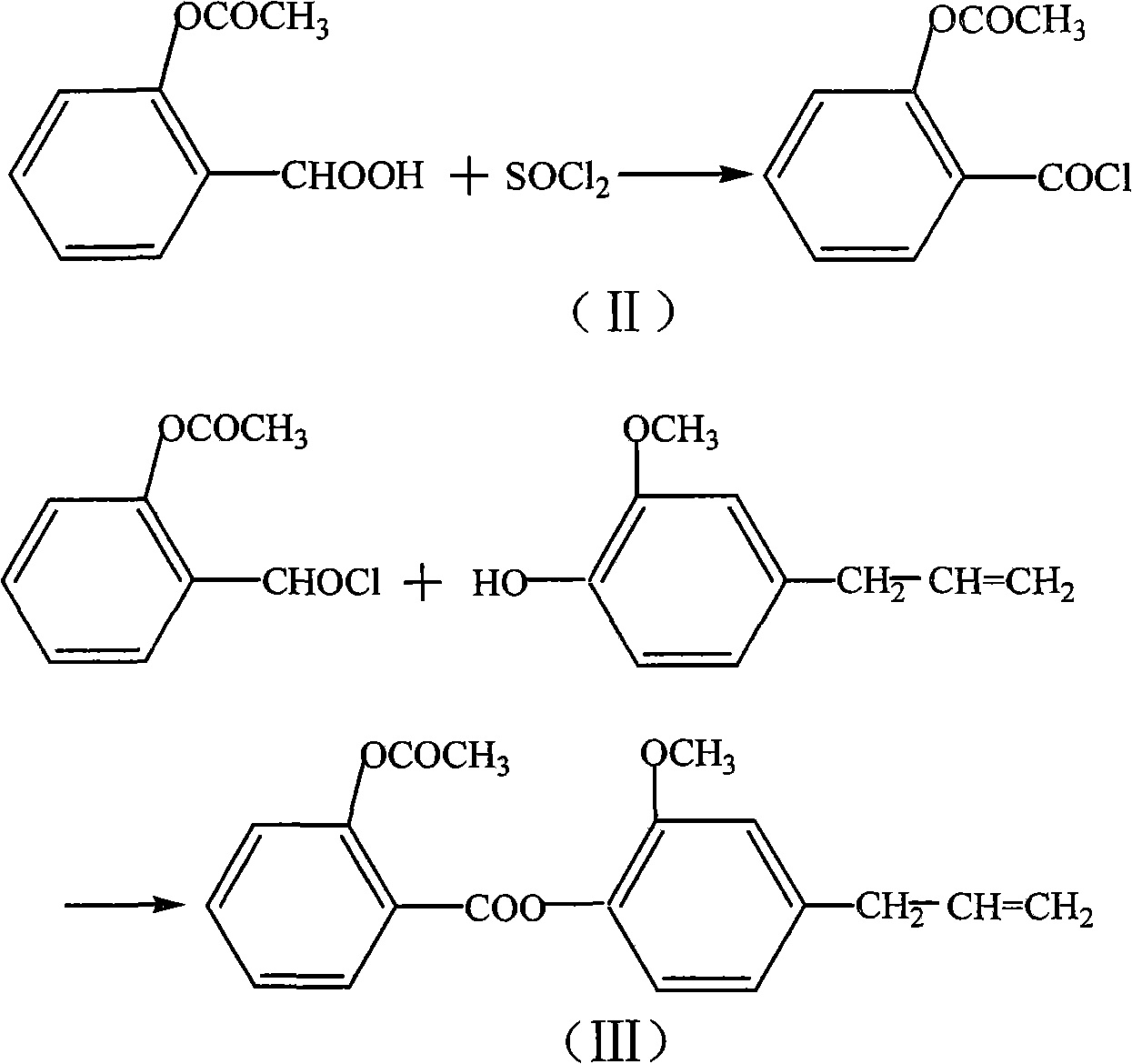
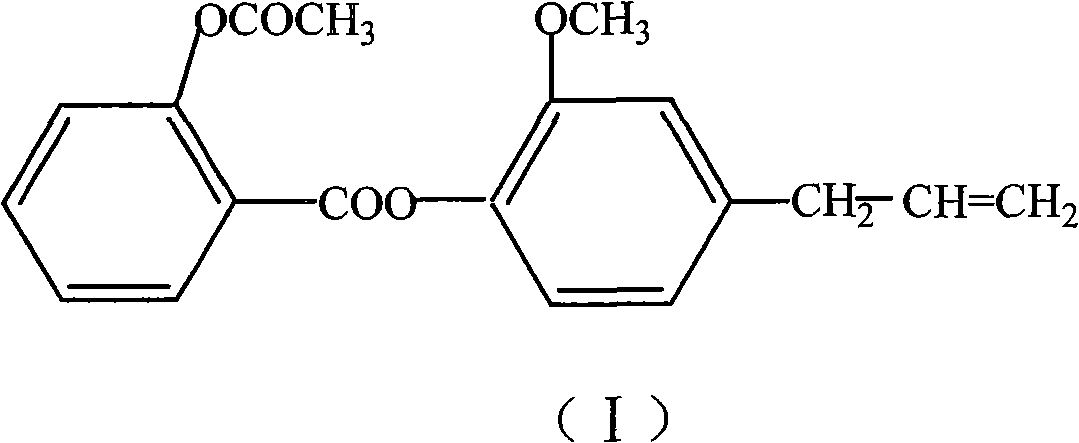
[0050] The concrete preparation route of eugenol aspirin ester is as follows:
[0051]
[0052] In a 250ml three-neck flask equipped with an electromagnetic stirrer and a thermometer, add 3.6g of aspirin, 1ml of DMF, and 2.8g of thionyl chloride, stir and react at 60-75°C for 2-3 hours, and evaporate under reduced pressure For unreacted thionyl chloride, the resulting acid chloride was dissolved in 8ml of benzene or 8ml of chloroform for use. Add 3.3g eugenol and 5ml water into the reaction flask, add 16ml 5% NaOH solution and PEG-1000 at 5-10°C, stir, add the above-mentioned acid chloride solution to be used after 0.5 hours, and continue the reaction for 2-5 Hour. After static separation, the organic phase was concentrated under reduced pressure to obtain a solid, which was recrystallized from methanol to obtain a white crystalline product with a yield of 65% and a melting point of 71-72°C.
[0053] The present invention uses the eugenol aspirin ester medicinal compound ...
Embodiment 1
[0055] Prescription: Eugenol aspirin ester 25mg
[0056] Medium Chain Triglycerides 34-50mg
[0057] Soy Lecithin 20-30mg
[0058] Macrogol-12-hydroxystearate 20-30mg
[0059] Absolute ethanol 30-40mg
[0060] PEG-400 10-20mg
[0061] Water 180-300ml
[0062] Preparation method: dissolve eugenol aspirin ester in medium-chain triglycerides containing absolute ethanol, PEG-400, soybean lecithin, polyethylene glycol-12-hydroxystearate, and stir overnight at 37°C until equilibrium; By titrating the oil phase with water, a uniform and stable microemulsion of eugenol aspirin ester can be formed.
Embodiment 2
[0064] Prescription: Eugenol aspirin ester 25mg
[0065] Phospholipids 40-60mg
[0066] Cholesterol 10-40mg
[0067] Chloroform 20-30mg
[0068] water 2-3ml
[0069] Preparation method: Dissolve eugenol aspirin ester in a chloroform solution containing phospholipids and cholesterol, and evaporate the chloroform to form a phospholipid film; dissolve the phospholipid film with water, and form uniform liposomes through cell crushing and ultrasonication.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com