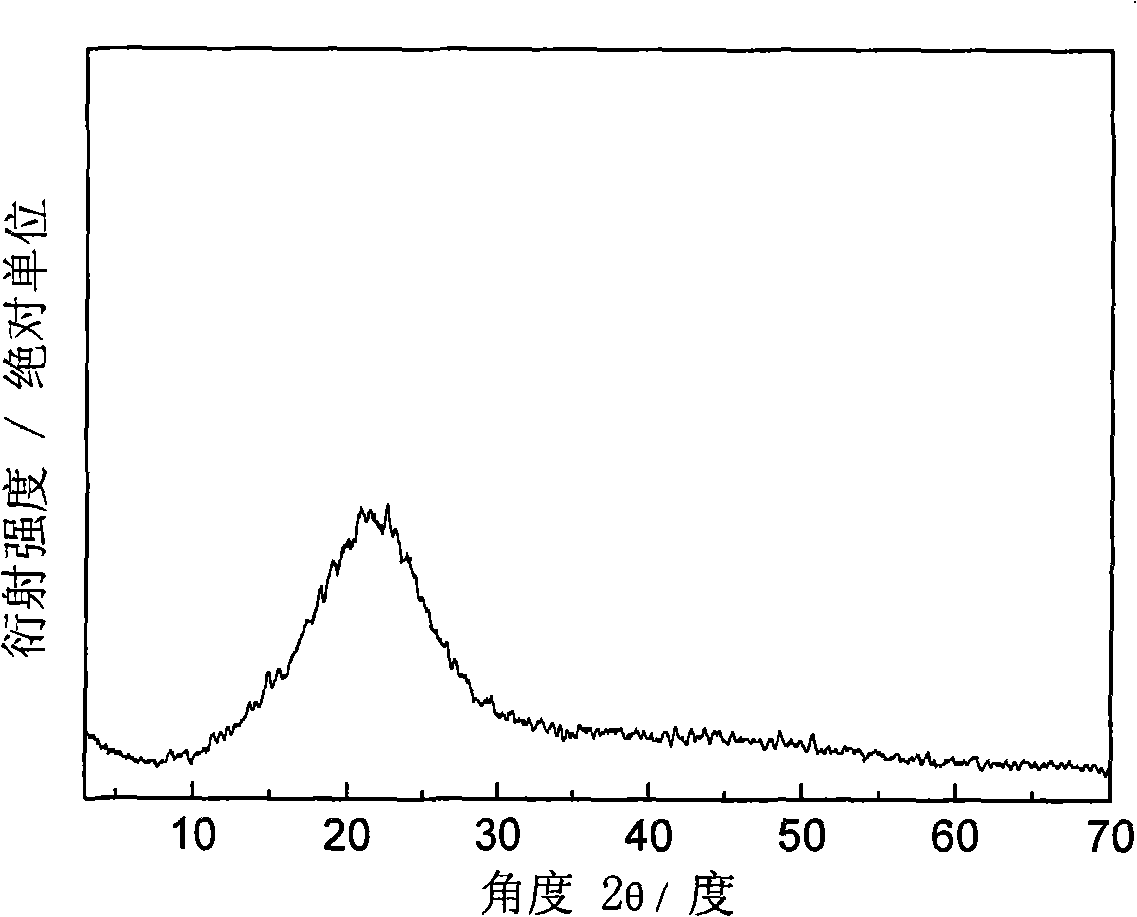
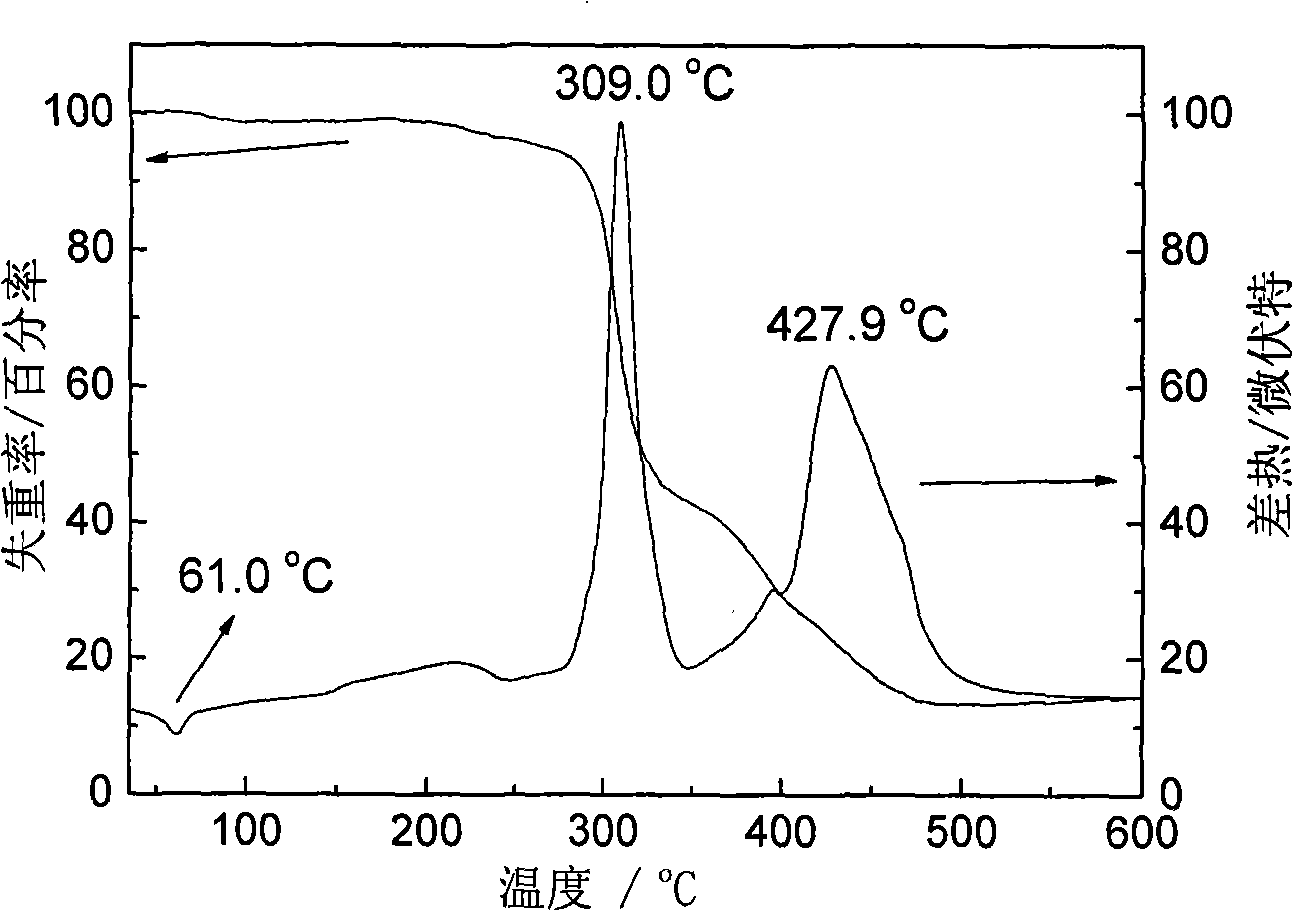
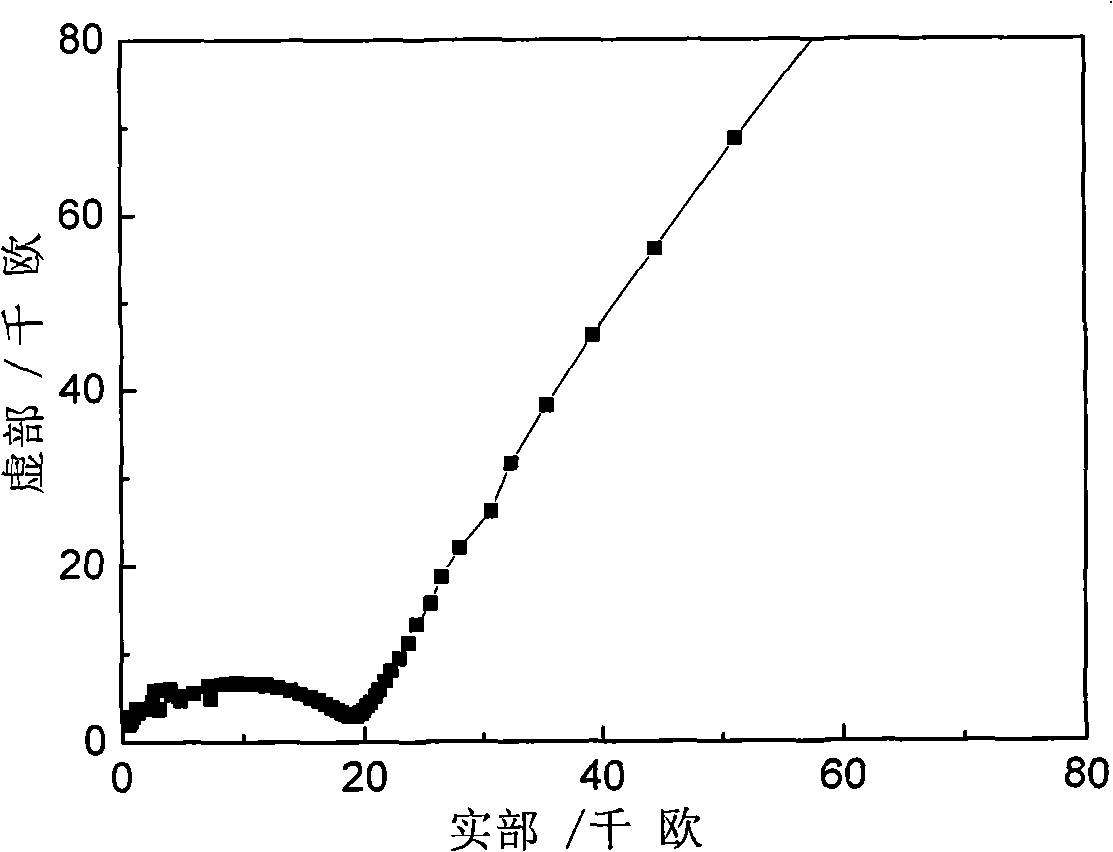
Inorganic/organic nano composite solid electrolyte and method of preparing the same
A solid electrolyte and nanocomposite technology, which is applied to batteries with solid electrolytes, solid electrolytes, electrolytes, etc., can solve the problems of reducing crystallinity, unable to meet lithium ion transport, thermal stability and mechanical properties need to be further improved, etc. The effect of good thermal stability, high room temperature conductivity and lithium ion migration number
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The experimental water was used to remove CO from the freshly boiled 2 of secondary deionized water, reacted at N 2 under atmosphere. Weigh 10.24g of Mg(NO 3 ) 2 ·6H 2 O and 7.50g of Al(NO 3 ) 3 9H 2 O, dissolved in 120mL to remove CO 2 deionized water to make a 0.5mol / L mixed solution (Mg 2+ with Al 3+ The molar ratio is 2:1), added dropwise to 80mL of lactic acid (LA) solution with a concentration of 0.5mol / L (LA and Mg 2+ The molar ratio is 4:1). At the same time, 40 mL of NaOH solution (NaOH and Mg 2+ The molar ratio is 5:1), stirring to keep the pH=11, and the resulting slurry is crystallized in a closed container at 100° C. for 10 hours to obtain lactic acid intercalated hydrotalcite. Lactic acid intercalated hydrotalcite with deCO 2 Wash with deionized water, then centrifuge at a high speed of 10,000 rpm for 5 minutes, remove the supernatant, and precipitate the solid with deCO 2 Continue high-speed centrifugation after washing with deionized water,...
Embodiment 2
[0029] The experimental water was used to remove CO from the freshly boiled 2 of secondary deionized water, reacted at N 2 under atmosphere. Weigh 2.97g of Zn(NO 3 ) 2 ·6H 2 O and 1.875g of Al(NO 3 ) 3 9H 2 O dissolved in 150mL to remove CO 2 deionized water to prepare a mixed solution with a concentration of 0.1mol / L (Zn 2+ with Al 3+ molar ratio of 2:1), was added dropwise to 50 mL of lactic acid (LA) solution with a concentration of 0.4 mol / L (LA and Zn 2+ The molar ratio is 2:1). At the same time, 80 mL of NaOH solution with a concentration of 1.0 mol / L (NaOH and Mg 2+ The molar ratio is 8:1), stirring to keep the pH=12, and the obtained slurry is crystallized in a closed container at 40° C. for 4 hours to obtain lactic acid intercalated hydrotalcite. Lactic acid intercalated hydrotalcite with deCO 2 Wash with deionized water, then centrifuge at a high speed of 8000 rpm for 10 minutes, remove the supernatant, and precipitate the solid with deCO 2 Continue hig...
Embodiment 3
[0033] The experimental water was used to remove CO from the freshly boiled 2 of secondary deionized water, reacted at N 2 under atmosphere. Weigh 23.28g of Co(NO 3 ) 2 ·6H 2 O and 7.50g of Al(NO 3 ) 3 9H 2 O dissolved in 100mL to remove CO 2 1.0mol / L mixed solution (Co 2+ with Al 3+ The molar ratio is 4:1), added dropwise to 100 mL of lactic acid (LA) solution with a concentration of 4.80 mol / L (LA and Co 2+ The molar ratio is 6:1). At the same time, 80 mL of NaOH solution (NaOH and Co 2+ The molar ratio is 5:1), stirring to maintain pH=10. The resulting slurry is crystallized at 80° C. for 16 hours in a closed container to obtain lactic acid intercalated hydrotalcite. Lactic acid intercalated hydrotalcite with deCO 2 Wash with deionized water, then centrifuge at 10,000 rpm for 15 minutes at a high speed, remove the supernatant, and precipitate the solid with deCO 2 Continue high-speed centrifugation after washing with deionized water, repeat the above-mentione...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical conductivity | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com