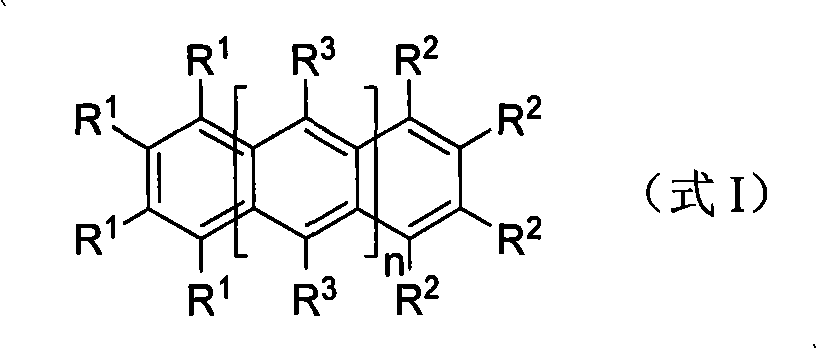
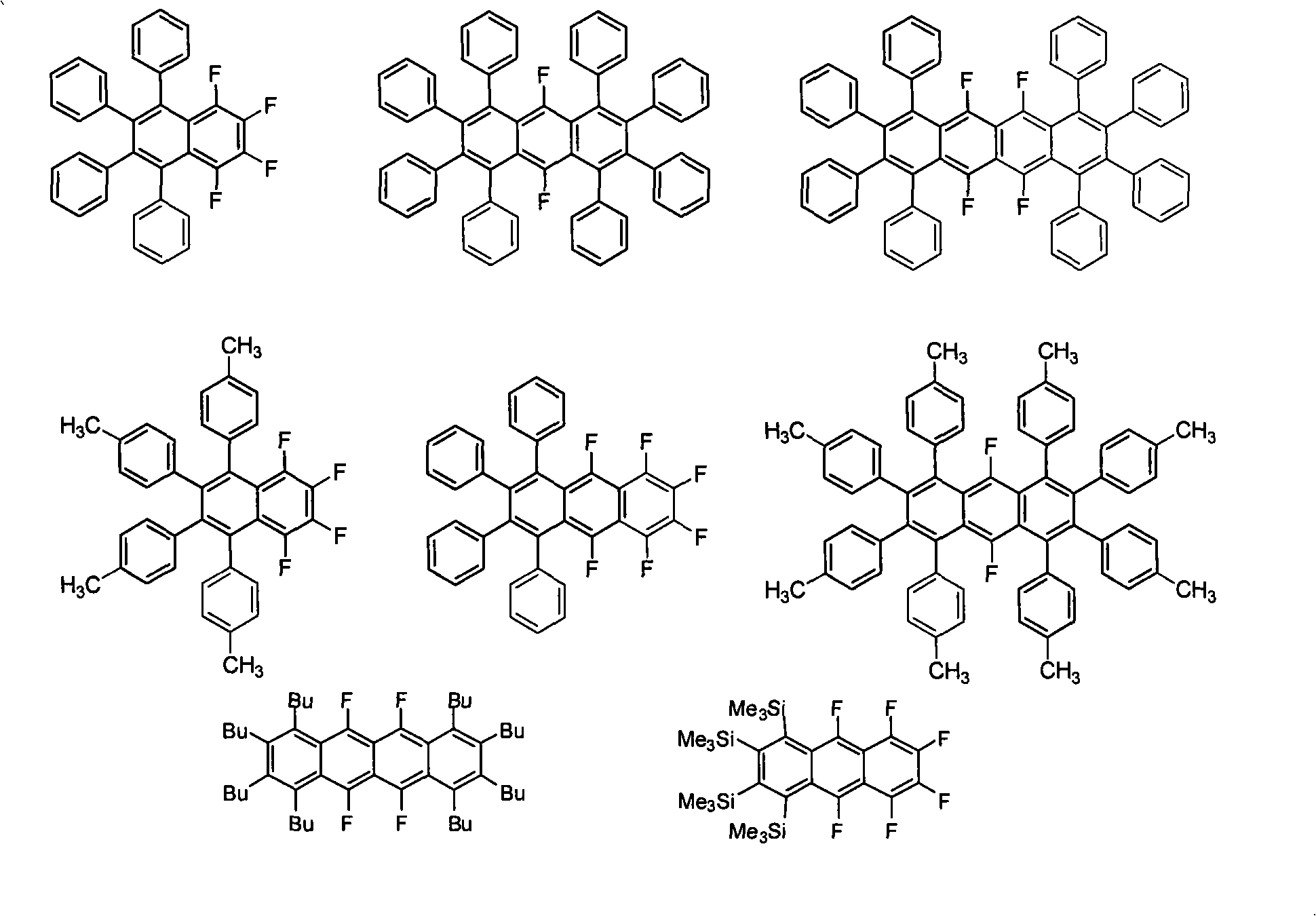
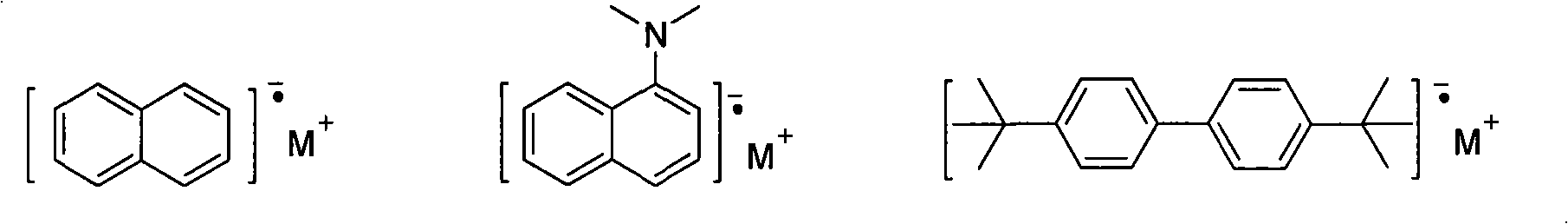Polysubstituted acene derivative and preparation thereof
A technology of benzene derivatives and multi-substitution, applied in the field of multi-substituted acene derivatives and preparation thereof, can solve the problems of increased operation complexity, unstable preparation, cumbersome steps and the like, and achieves mild conditions, simple process and easy operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0022] The preparation method of multi-substituted acene derivatives of the present invention is as follows:
[0023] Stir and react 1,2-disubstituted acetylene derivatives with a certain amount of reducing agent in an organic solvent for 1 minute to 36 hours, and the reaction temperature is -78°C to 50°C; then, add polyhalogenated benzene derivatives and stir the reaction From 1 minute to 36 hours, the reaction temperature is -78°C to 50°C; finally, the reaction is quenched with water, an aqueous inorganic salt solution, or acid, and the target product is obtained by separation and purification.
[0024] By simply adjusting the ratio between 1,2-disubstituted acetylene derivatives and polyhalogenated benzene derivatives, "asymmetry" (such as Examples I, IV, V) or "symmetry" (such as Examples II, III, VI) reaction product of structure. When the ratio between polyhalogenated benzene derivatives and 1,2-disubstituted acetylene derivatives is greater than or equal to 1:2, it is ...
Embodiment 1、1
[0028] Embodiment 1, 1,2,3,4-tetrafluoro-5,6,7,8-tetraphenylnaphthalene (1,2,3,4-tetrafluoro-5,6,7,8-tetraphenylnaphthalene, I) preparation of
[0029] Take a 50ml two-necked flask, add lithium (18mg, 2.63mmol), naphthalene (0.89g, 2.65mmol), tetrahydrofuran (5ml), stir and react at 25°C for 4 hours to obtain a naphthalenelithium solution. To this solution was added a solution of tolanylacetylene (0.31g, 1.75mmol) in tetrahydrofuran (2ml) at 25°C, and the reaction was stirred for 20 minutes, then hexafluorobenzene (0.40g, 2.17mmol) was added, stirred for 1 hour, and then Add 1 ml of saturated aqueous ammonium chloride solution and stir for 5 minutes to stop the reaction. Extracted with ether, dried over anhydrous magnesium sulfate, and rotary evaporated under reduced pressure, the obtained crude product was treated with SiO 2 Column chromatography (petroleum ether rinse at 60-90°C), and recrystallization (tetrahydrofuran / ethanol) purified to obtain 131 mg (0.26 mmol) of the ...
Embodiment 2、9
[0032] Example 2, 9,10-difluoro-1,2,3,4,5,6,7,8-octaphenylanthracene (9,10-difluoro-1,2,3,4,5,6, 7,8-octaphenylanthracenel, II) Preparation
[0033] Take a 50ml two-necked flask, add lithium (104mg, 15.04mmol), naphthalene (1.92g, 14.96mmol), tetrahydrofuran (8ml), and stir at 25°C for 4 hours to obtain a naphthalenelithium solution. To this solution was added a solution of tolanylacetylene (1.78g, 9.98mmol) in tetrahydrofuran (4ml) at 25°C, and the reaction was stirred for 15 minutes, then hexafluorobenzene (0.46g, 2.49mmol) was added, stirred for 1 hour, and then Add 1 ml of saturated aqueous ammonium chloride solution and stir for 5 minutes to stop the reaction. Extracted with dichloromethane, dried over anhydrous magnesium sulfate, and rotary evaporated under reduced pressure, the obtained crude product was purified by recrystallization (dichloromethane / n-hexane, 3 / 1) to obtain product 0.41g (0.50mmol), and the yield was 20 %.
[0034] Melting point: >300°C
[0035] HR...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com