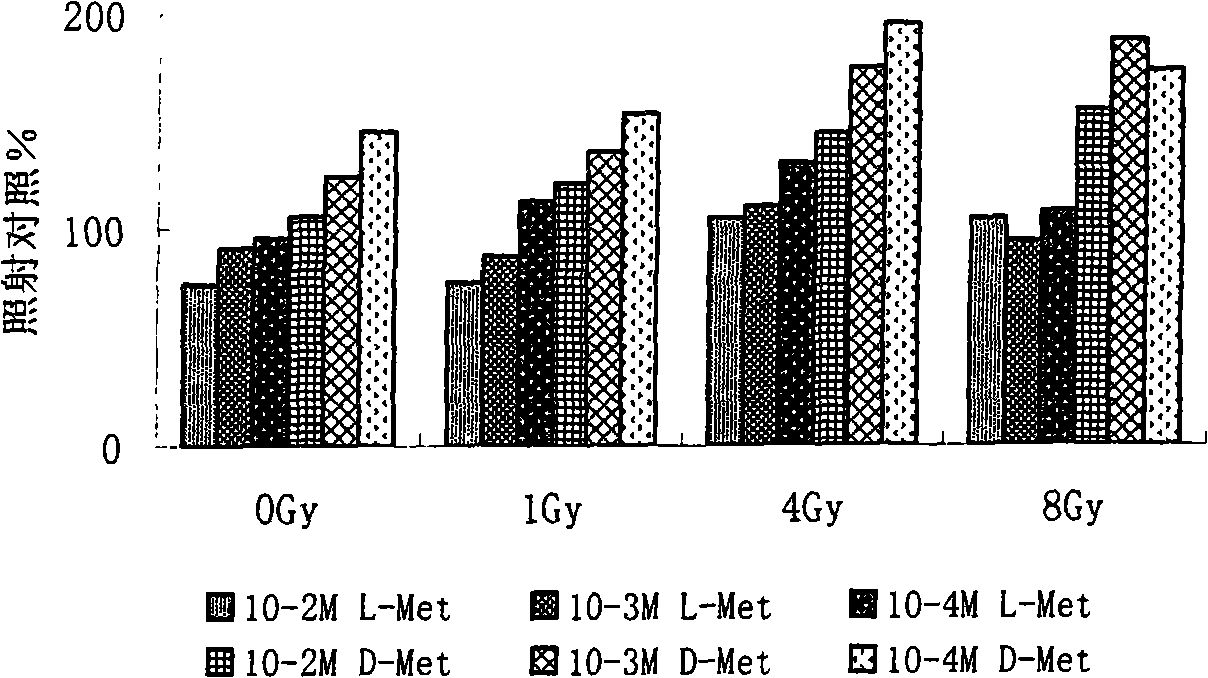Application of clock wise D-methionine in preparing the medicine for preventing and curing the myelosuppression induced by radiation
A technology of D-methionine and bone marrow suppression, which is applied in the direction of drug combinations, pharmaceutical formulas, and medical preparations containing active ingredients, etc., which can solve the problems of uncertain curative effect and high cost of use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
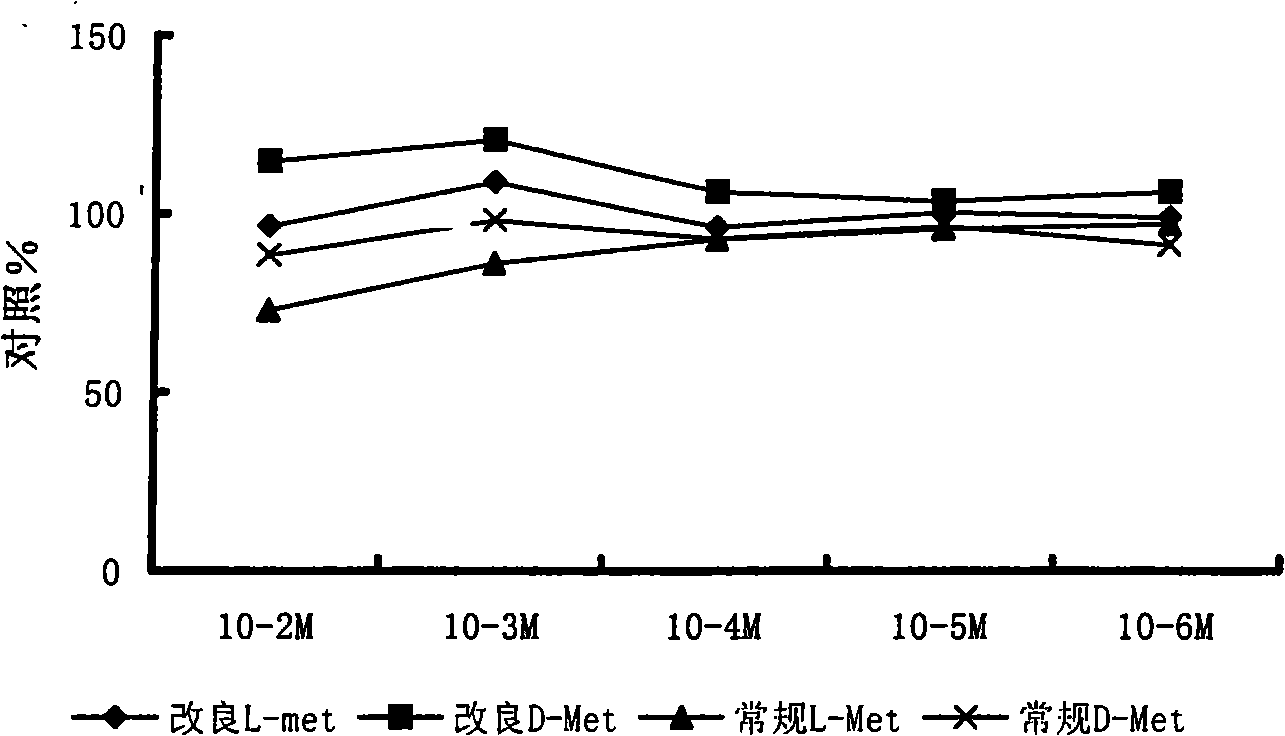
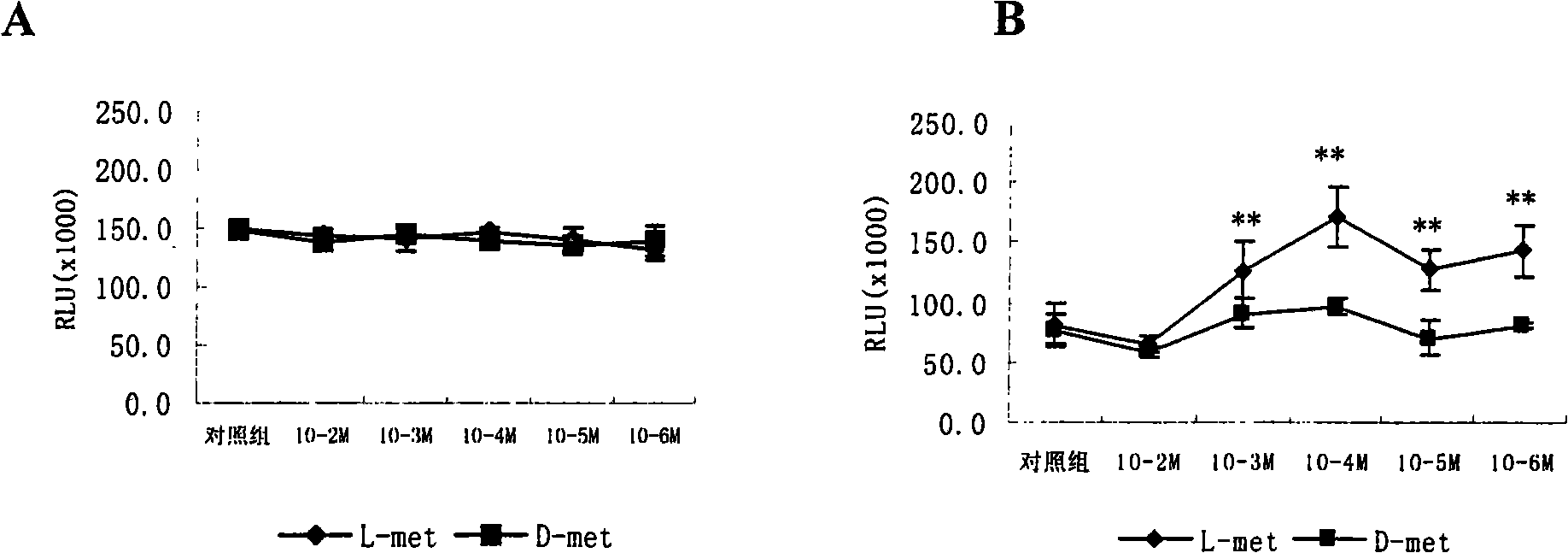
Image
Examples
Embodiment 1
[0080] D-met100mg, lactose 50mg, microcrystalline cellulose 80mg, starch 50mg, hydroxymethylcellulose 40mg, magnesium stearate 5mg. Pass the active ingredients, lactose, starch, and microcrystalline cellulose through a 100-mesh sieve, and mix thoroughly, add 2% carboxymethylcellulose aqueous solution to the above mixed powder and mix, pass through a 20-mesh sieve to make soft materials, and obtain wet granules Dry at 45-55°C, add sodium carboxymethyl starch and magnesium stearate to the above-mentioned dry granules and press into tablets.
Embodiment 2
[0082] D-met 100mg, Shengbaixin 500mg, add 4g (lactose-microcrystalline fiber 5:1), magnesium stearate 1%, granulate with 70% ethanol, and compress into tablets.
Embodiment 3
[0084] D-met 10g, mannitol 60g, add 1000ml water for injection to dissolve, add water for injection to 2000ml, add appropriate amount of activated carbon to remove heat source, filter with 0.2um microporous membrane, fill, freeze-dry, and prepare 2000 freeze-dried powder injections . Specifications: 70mg / bottle, for intravenous injection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com