Ellagic acid compounds, preparation thereof and applications in anticancer drugs
A compound, the technology of ellagic acid, applied in the field of anticancer drugs and its preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] The extraction and separation methods of two ellagic acid compounds 3,3'-di-O-methylellagic acid and 3,3'-di-O-methylellagic acid-4'-O-β-D-xylopyroside:
[0045] ① Jiuniuzao medicinal materials were collected from Taibai County, Shaanxi Province in October 2005. After natural air-drying, they were crushed. Soak 9kg of crushed Jiuniuzao in 85% ethanol for 12h, reflux extraction 3 times, 4h each time, and combine the extracts , concentrated under reduced pressure to extract;
[0046] ② Add an appropriate amount of water to the extract, then extract with petroleum ether, chloroform, and ethyl acetate, and recover the solvent to obtain 20g, 89g, and 170g of petroleum ether, chloroform, and ethyl acetate respectively;
[0047] ③The chloroform part is subjected to silica gel column chromatography, first use petroleum ether-ethyl acetate and then use chloroform-methanol as the eluent to carry out gradient elution, and collect 150ml of each part of the eluent in equal amounts, ...
Embodiment 2
[0050] Structural identification of two ellagic acid compounds 3,3'-di-O-methylellagic acid and 3,3'-di-O-methylellagic acid-4'-O-β-D-xylopyroside
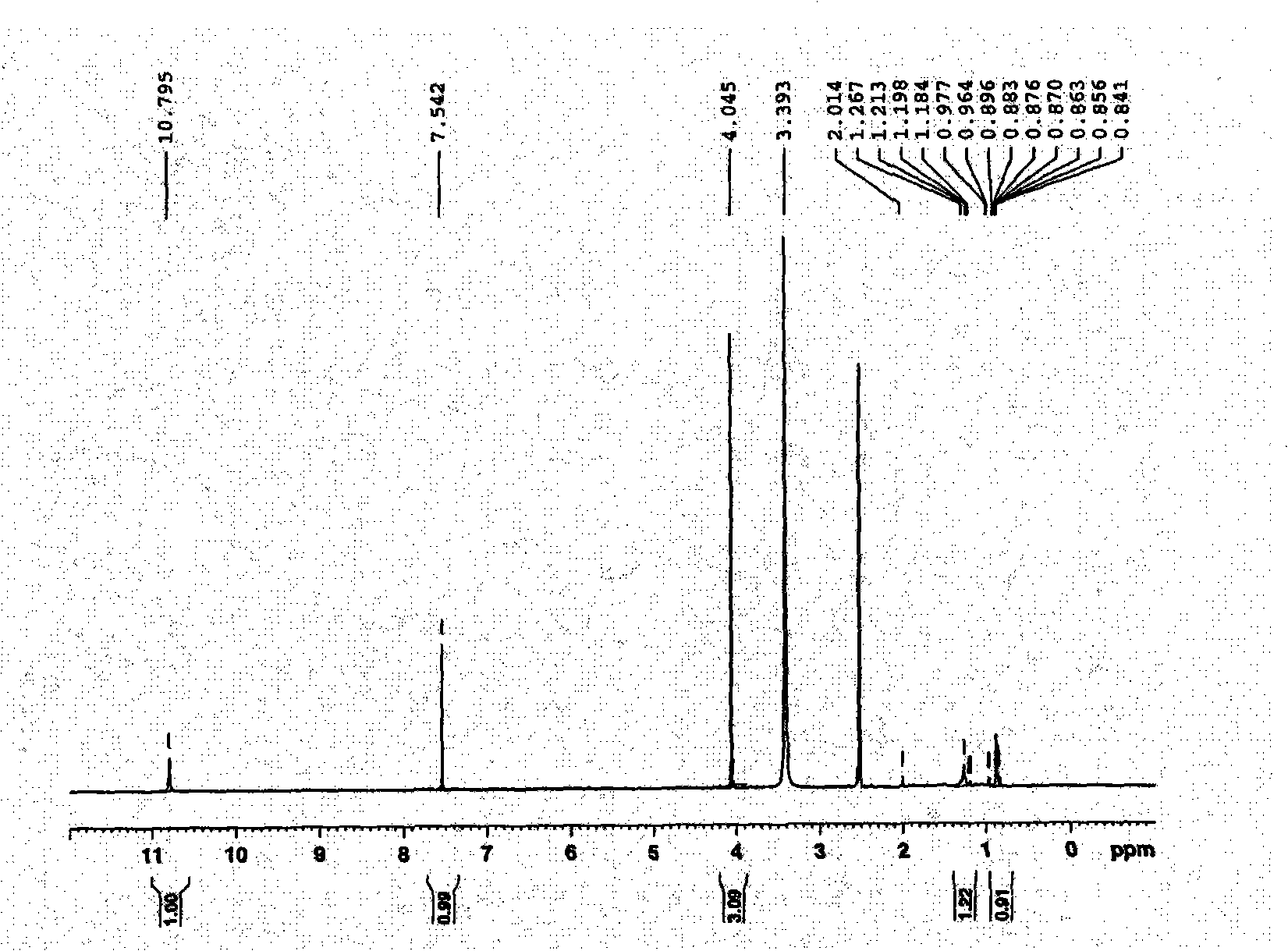
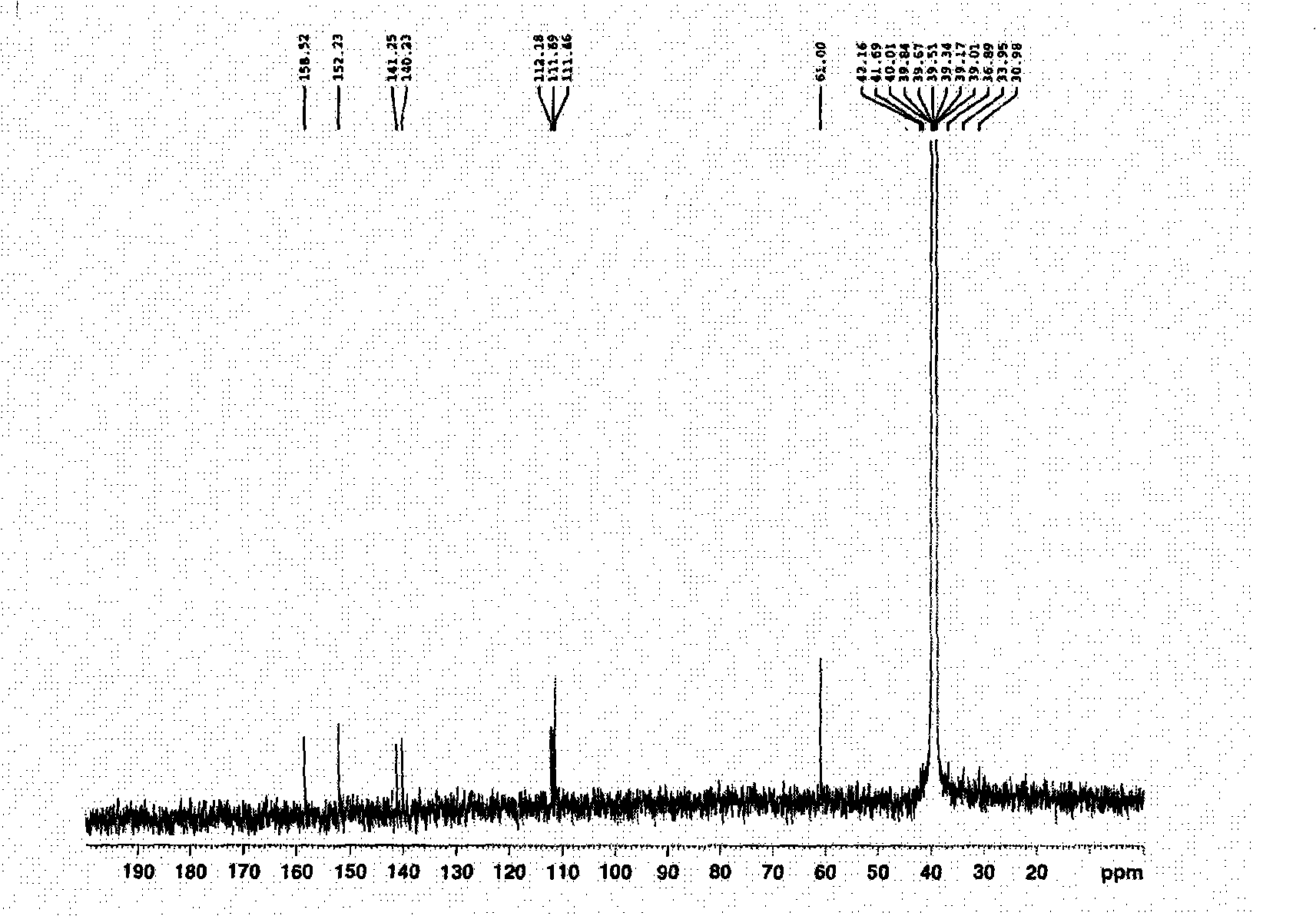
[0051] The crystals precipitated in the eluent during partial column chromatography of ethyl acetate are light yellow powder crystals with a melting point of 332-334°C, MS showing a molecular weight of 344, and a molecular formula of C 16 h 10 o 8 . pass 1 H-NMR and 13 C-NMR spectrum (see attached figure 1 And attached figure 2 ) hints that it is an ellagic acid substance, and its chemical structural formula is as follows:
[0052]
[0053] 3,3'-di-O-methylellagic acid
[0054] attached figure 1 1 The spectral data and attribution in the H-NMR spectrum are as follows:
[0055] 1 H-NMR (DMSO-d 6 )δppm: 4.05 (6H, s, 2×OCH 3 ), 7.54 (2H, s, H-5, H-5'), 10.79 (2H, s, OH-4, OH-4').
[0056] attached figure 2 13 C-NMR spectrum spectrum data and attribution are as follows:
[0057] 13 C-NMR (DMSO-d 6 )δppm: 111.46...
Embodiment 3
[0070] Anti-liver cancer effects of 3,3'-di-O-methylellagic acid and 3,3'-di-O-methylellagic acid-4'-O-β-D-xylopyroside
[0071] 1. Cell Culture and Monomer Compounds
[0072] The human liver cancer cell line SMMC-7721 was provided by the Medical Research and Experimental Center of Xi'an Jiaotong University. The cells were cultured in RPMI-1640 medium containing 10% calf serum at 37°C and 5% CO 2 In a saturated humidity incubator, every 2 to 3 days when the cells were confluent, they were digested and passaged with 0.25% trypsin, and the cells in the logarithmic growth phase were used for the experiment. The two monomer compounds are 3,3'-di-O-methylellagic acid and 3,3'-di-O-methylellagic acid-4'-O-β- D-xylopyroside.
[0073] 2. Test method
[0074] Determination of ellagic acid substances 3,3'-di-O-methylellagic acid and 3,3'-di-O-methylellagic acid-4'-O-β-D-xylopyroside in Jiuniuzao by MTT method (with DMSO Dissolution) inhibited the growth of liver cancer cells.
[00...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com