Fluorescent probe for detecting generation of nitric oxide and use thereof
A fluorescent probe, nitric oxide technology, applied in the field of biological detection and clinical medical detection, can solve the problems of inability to realize real-time three-dimensional detection of NO, lack of specificity of NO detection, slow progress of NO detection, etc., and achieve enhanced fluorescence intensity. , good selectivity, good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Embodiment 1: the common synthetic route of compound 3a-Cu, 3b-Cu, 3c-Cu, 3d-Cu, 3h-Cu
[0051] Dissolve 300mmol of substituted salicylaldehyde (1a~1d) in 125ml of methanol under stirring, then add 150mmol of substituted o-phenylenediamine (2a or 2b), stir at room temperature for 1 hour, and generate the corresponding precipitate, which is collected by filtration The precipitate was washed with methanol and dried in vacuo to obtain ligands Aa~Ah.
[0052] Dissolve 13mmol of ligands Aa~Ah in 100ml of chloroform, and then add 2.5g of Mn(II)Cl 2 4H 2 O was dissolved in the prepared solution of 40ml ethanol to generate a brown solution, and then this solution was stirred for 12 hours under the situation of feeding air to obtain a brown solution, stirred at room temperature for 24 hours and concentrated to generate a brown precipitate, which was filtered out. Wash with DMF, then with a small amount of ethanol, and dry in vacuum to obtain the corresponding trivalent mangane...
Embodiment 2
[0067] Embodiment 2: the common synthetic route of compound 4a, 4b, 4c, 4d, 4e, 4f and its copper complex:
[0068] Dissolve 300 mmol of substituted salicylaldehyde (1a-1f) in 125 ml of methanol under stirring, then add 150 mmol of o-naphthalene diamine (2), and stir at room temperature for 1 hour to generate a corresponding precipitate, which is collected by filtration. Washed with methanol and dried in vacuum to obtain the ligands Da~Df.
[0069] Dissolve 13mmol of ligands Da~Df in 100ml of chloroform, and then add 2.5g of Mn(II)Cl 2 4H 2 O was dissolved in the prepared solution of 40ml ethanol to generate a brown solution, and then this solution was stirred for 12 hours under the situation of feeding air to obtain a brown solution, stirred at room temperature for 24 hours and concentrated to generate a brown precipitate, which was filtered out. Wash with DMF, wash with a small amount of ethanol, and dry in vacuum to obtain trivalent manganese complexes Ea-Ef.
[0070] Di...
Embodiment 3
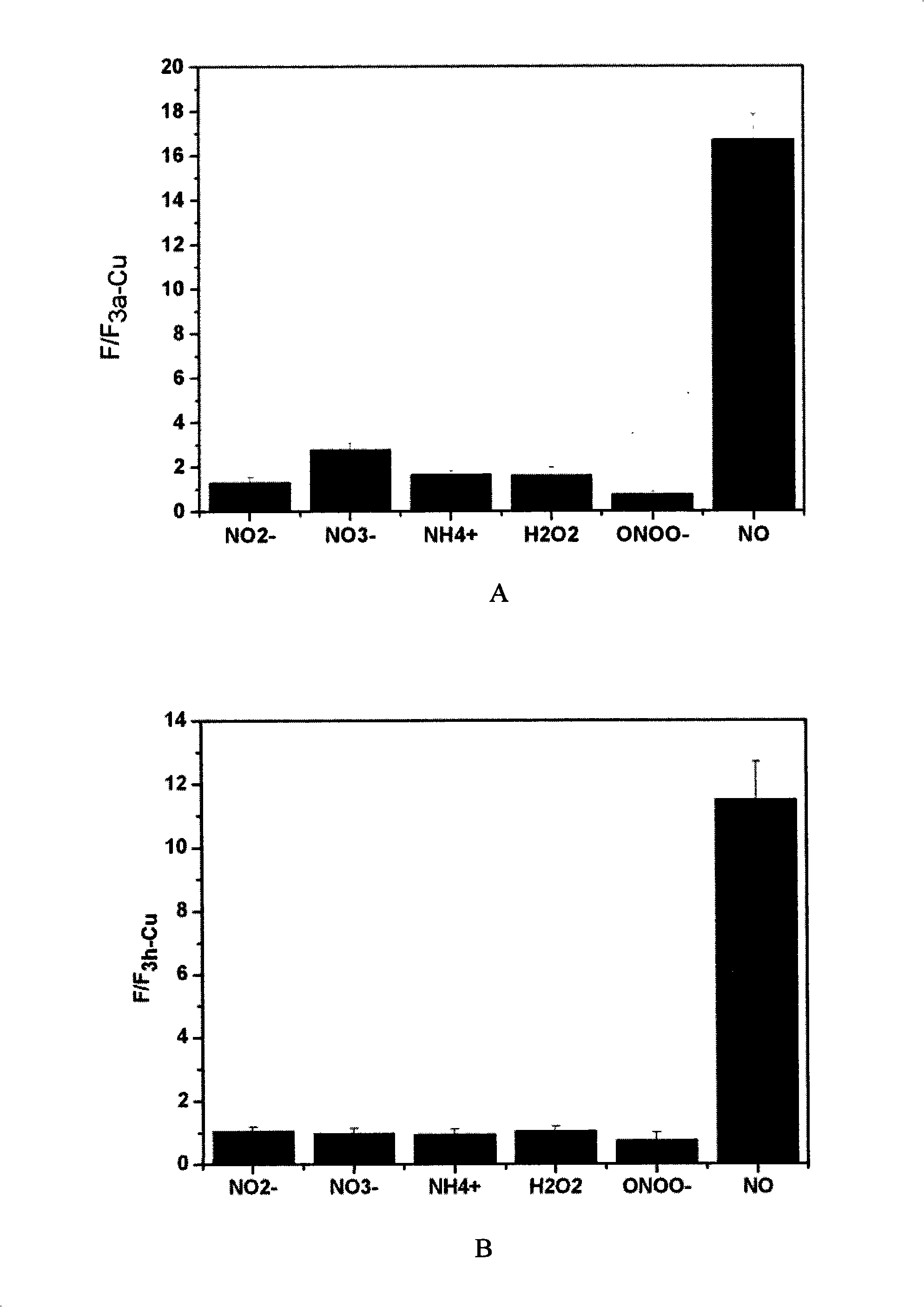
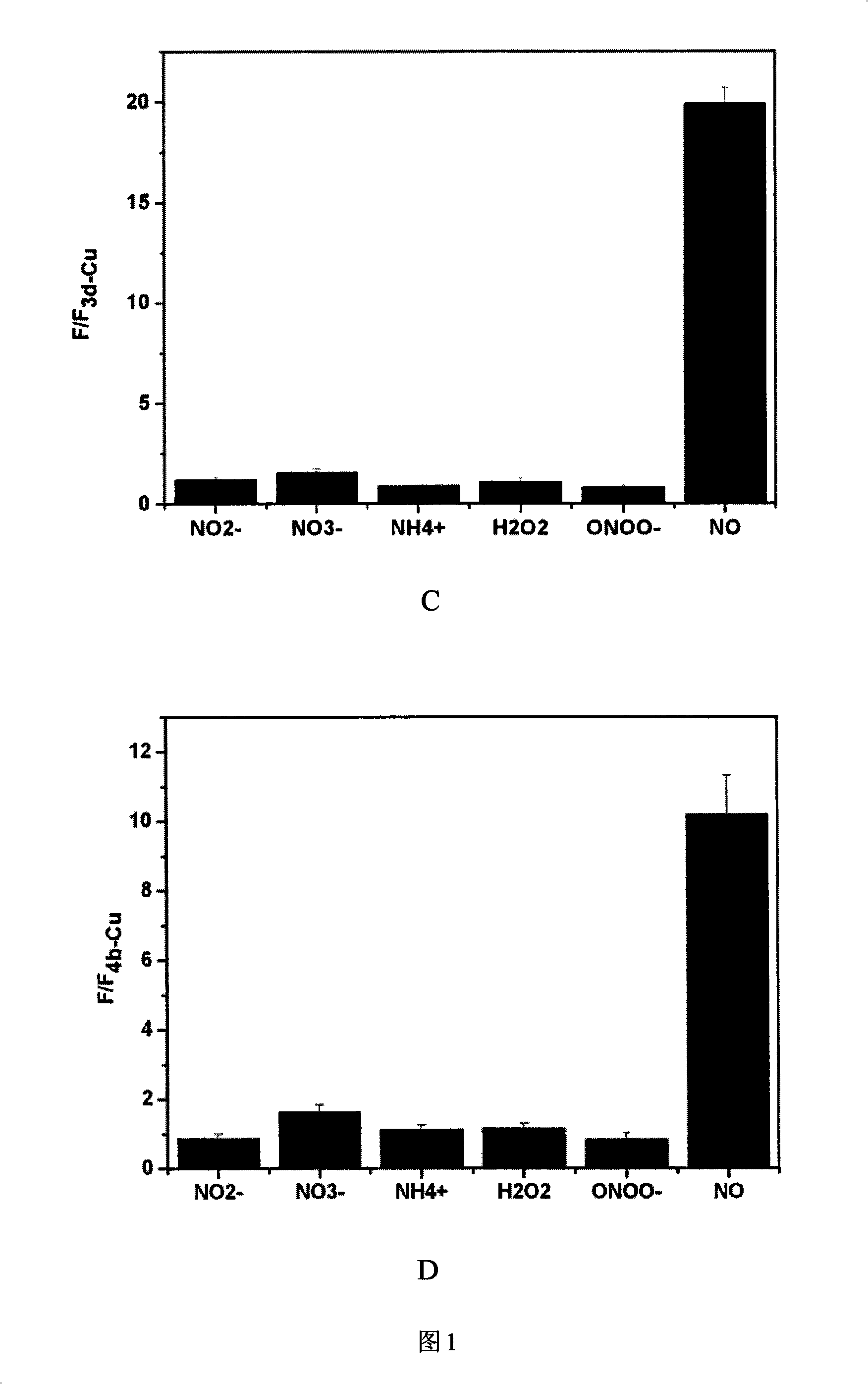
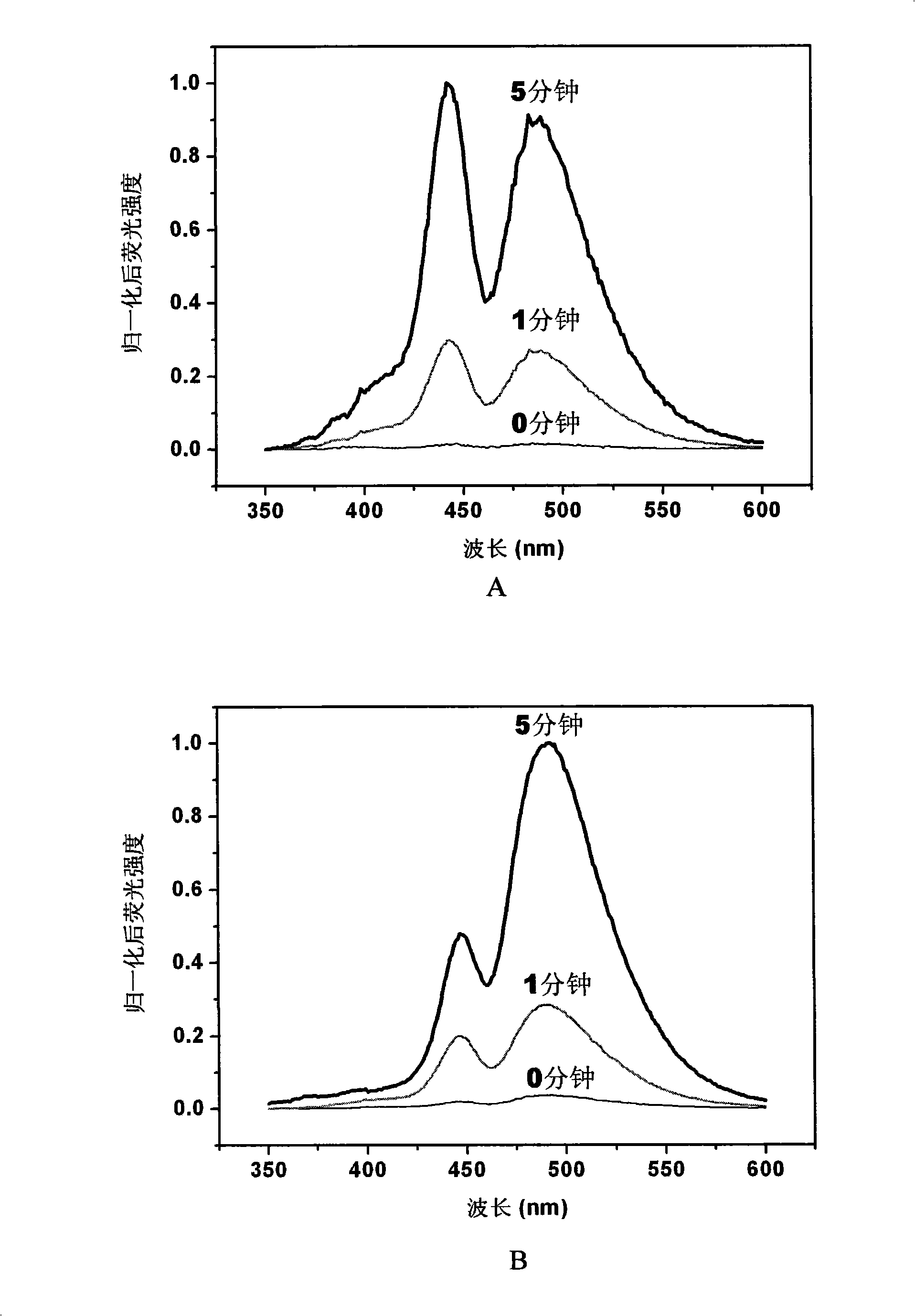
[0075] Embodiment 3: the selectivity of complex to NO
[0076] Take 3a-Cu, 3d-Cu, 3h-Cu, 4b-Cu and dissolve them in DMSO to a final concentration of 1 mmol / L. Add various copper complexes to the 96-well plate, respectively add nitrite, ammonium, nitrate, hydrogen peroxide, ONOO - , NO, and then add deionized water to all the samples, so that the probe concentration in each well is 100 μmol / L. Nitrite, Ammonium, Nitrate, Hydrogen Peroxide, ONOO - , the molar amount of NO is 100 times that of the corresponding probe, respectively. After reacting for 1 hour, the fluorescence intensity was measured on a microplate reader, and the fluorescence intensity after the reaction was divided by the fluorescence intensity of the original copper ion complex to normalize the fluorescence value. The results are shown in Figure 1, where A is 3a-Cu, B is 3d-Cu, C is 3h-Cu, and D is 4b-Cu.
[0077] It can be seen from the figure that the probes 3a-Cu, 3d-Cu, 3h-Cu, 4b-Cu have high selectivity...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com