Process for preparation of iron sulfide
A protective atmosphere and mixture technology, applied in the field of FeS preparation, can solve the problems of expensive raw material processing equipment, high control requirements, and poor process, and achieve the effect of easy production equipment, simple equipment, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
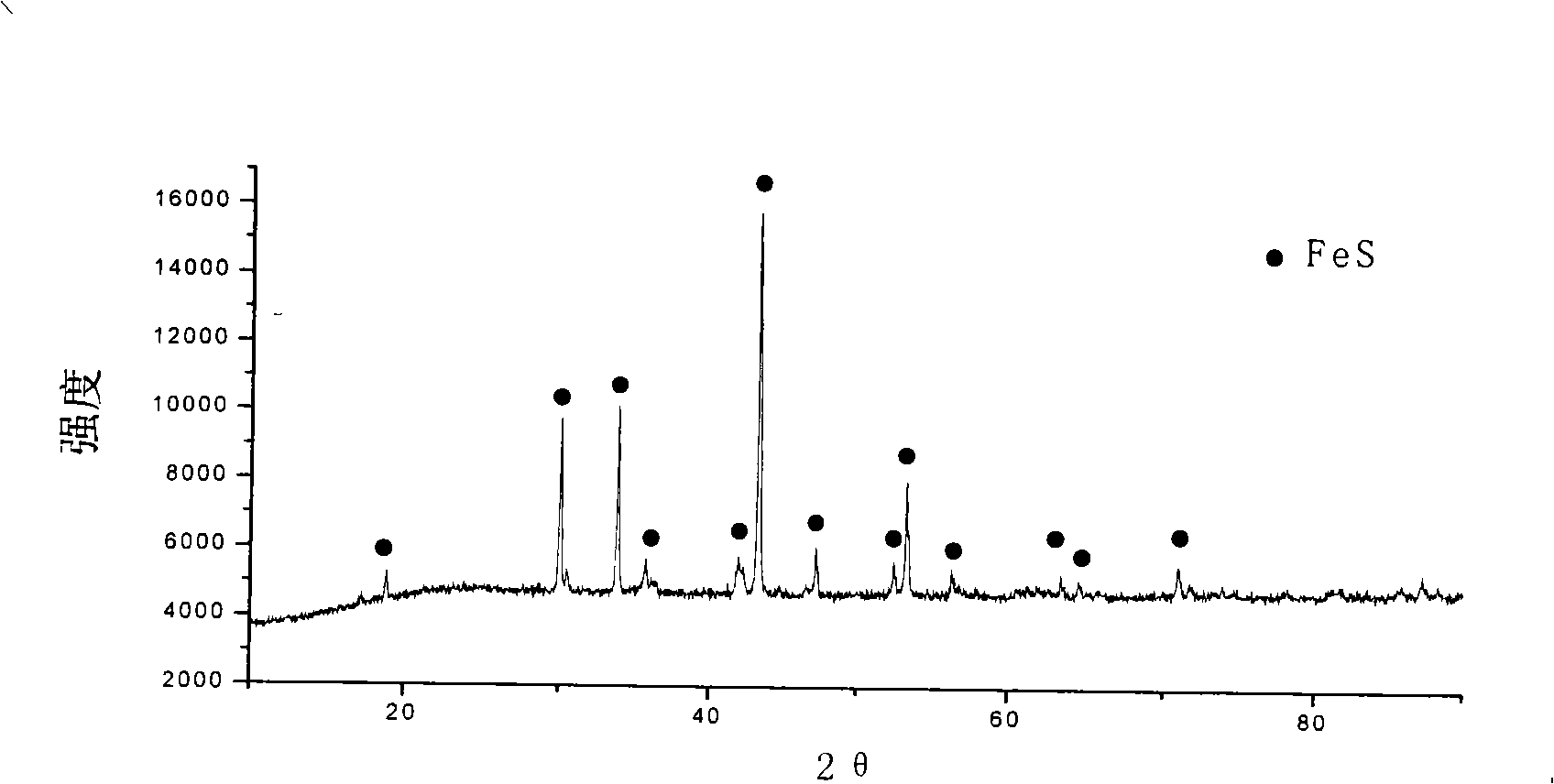
[0026] will analyze pure FeSO 4 ·7H 2 O5.117g is mixed with activated carbon 0.883g evenly, is packed in the quartz glass tube, after vacuumizing, at a pressure of 10 4 Under the protection of Pa nitrogen gas, put the quartz tube into the tubular resistance furnace, raise the temperature to 300°C at a rate of 10°C / min, keep it warm for 30min, and vacuum again, at a pressure of 1.5×10 4 Under the protection of nitrogen in Pa, the temperature was raised to 800°C at a rate of 7°C / min, and the product was naturally cooled after 6 hours of constant temperature to obtain the product. The purity of the product was >98.5%. It was FeS with a single phase space group of P63 / mmc. figure 1 .
Embodiment 2
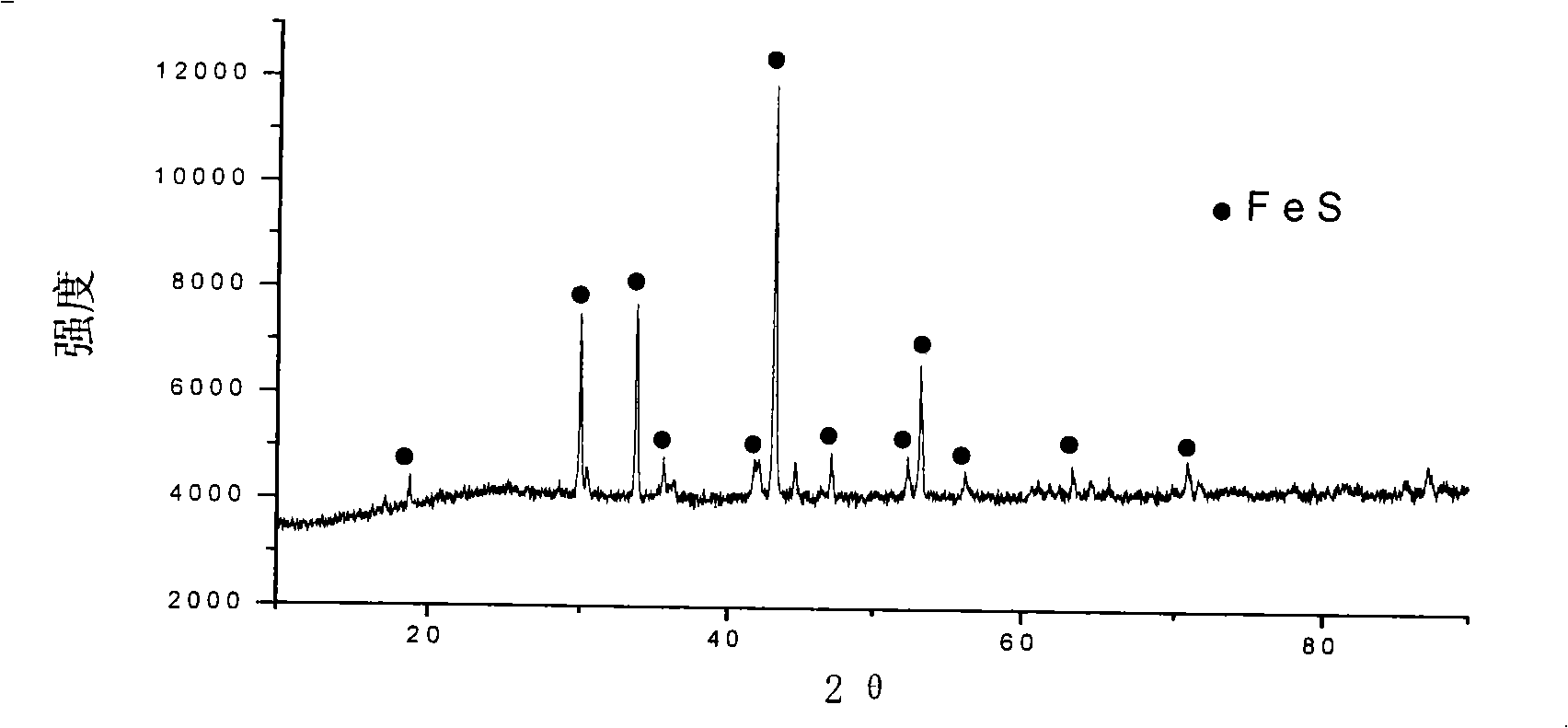
[0028] According to the same material and steps of the embodiment, the pure FeSO 4 ·7H 2 O5.001g is mixed with graphite 0.862g evenly, and the temperature is 120 ℃, and the vacuum is 133Pa under the condition of vacuum drying for 3 hours, after drying, the material is packed into a quartz glass tube, and after vacuuming, the 4 Under the protection of Pa nitrogen, put the quartz tube into the tubular resistance furnace, raise the temperature to 300°C at a rate of 13°C / min, keep it warm for 30min, and vacuum again, at 2×10 4 Under the protection of nitrogen in Pa, the temperature rises to 800°C at a rate of 8°C / min, the temperature is maintained for 6 hours, and then naturally cooled to obtain the product. The purity is the same as in Example 1. It is FeS with a single phase space group of P63 / mmc, XRD diffraction pattern See figure 2 .
Embodiment 3
[0030] will analyze pure FeSO 4 ·7H 2 Mix O5.000g with 0.723g of activated carbon evenly, put it into a quartz glass tube, and vacuumize it under a pressure of 2×10 4 Under the protection of Pa nitrogen, put the quartz tube into the tubular resistance furnace, raise the temperature to 300°C at a rate of 10°C / min, keep it warm for 40min, and vacuum again, at a pressure of 5×10 4 Under the protection of Pa nitrogen, the temperature was raised to 800° C. at a rate of 7° C. / min, kept at a constant temperature for 6 hours, and then naturally cooled to obtain the product. The purity was the same as in Example 1, and it was FeS with a single phase space group of P63 / mmc.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com