Preparation and use of galactosylated human serum albumin fused interferon
A technology of serum albumin and interferon, applied in the field of fusion interferon, can solve the problems of IFN not being able to target the liver and insufficient galactosylation, and achieve the effect of reducing treatment costs, reducing the degradation rate in the body, and reducing side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
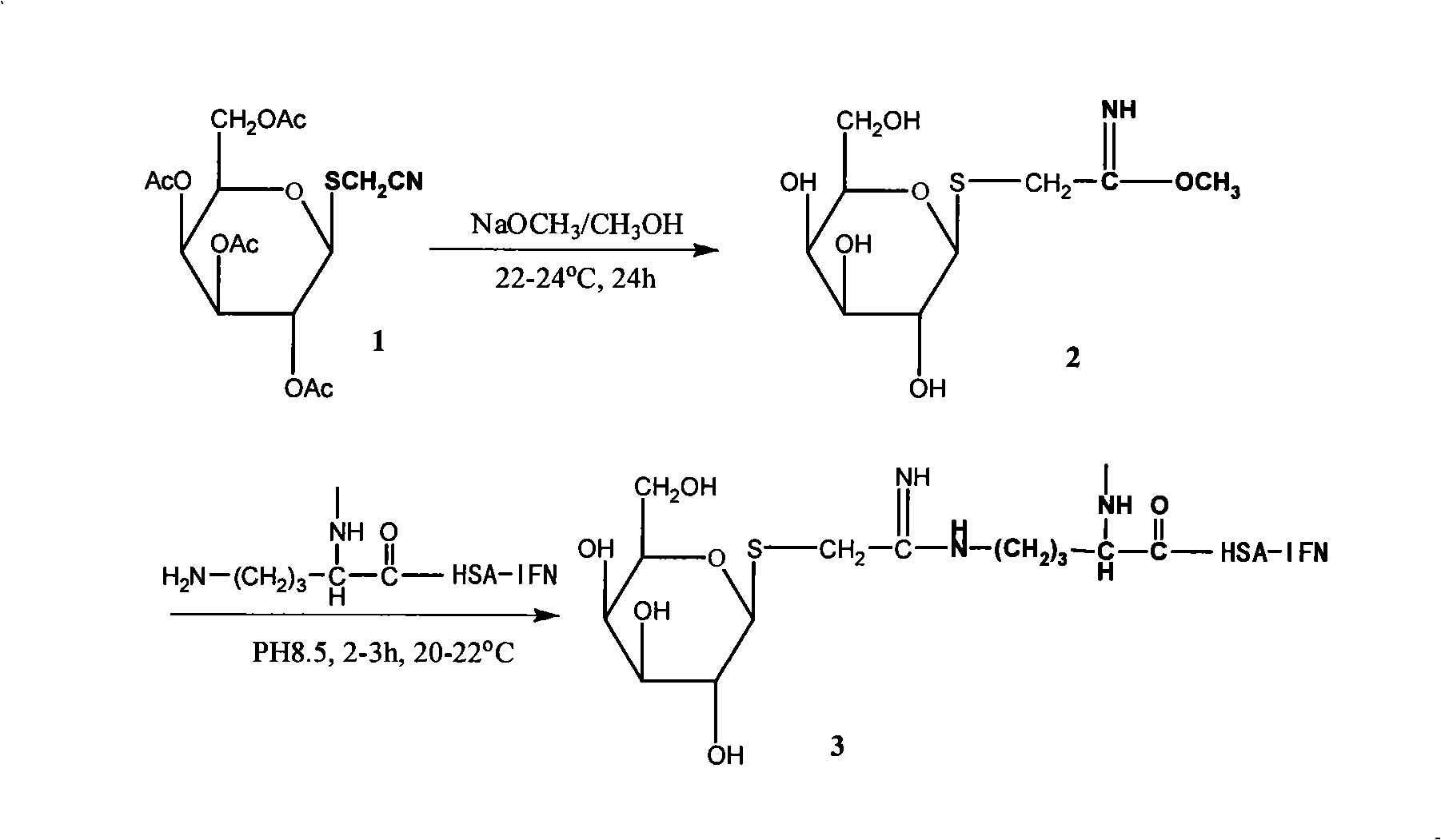
[0041] Example 1: Preparation of GHSA-IFNα2b
[0042] The reaction equation is:
[0043]
[0044] Compound 1 (0.28g, 0.69mmol) was dissolved in 10mL of anhydrous methanol, sodium methoxide (3.7mg, 0.069mmol) was added, and stirred at 20-22°C for more than 24 hours to obtain IME-thiogalactoside (compound 2, produced The yield is about 56%) and by-products (deacetylation products of compound 1), compound 2 need not be isolated.
[0045] Take 6mL of the above reaction solution, rotate to evaporate, remove the solvent, add 4.5mL of 50mg~900mg HSA-IFN solution prepared with 0.1mol / L pH8.5 boric acid buffer, stir and react at 20-22°C for 4 hours, then use PD-10 After column purification, the dialysis was continued with distilled water, and the dialysate was changed every 4 hours until no sugar was detected in the dialyzed fluid to obtain GHSA-IFNα2b (compound 3).
Embodiment 2
[0046] Example 2: Analysis and identification of GHSA-IFNα2b
[0047] The protein concentration of GHSA-IFNα2b was determined by the Lowry method, the protein was quantified, subpackaged, freeze-dried, and stored at -30°C; the sugar concentration of GHSA-IFNα2b was measured by the phenol-sulfuric acid method, and the average sugar concentration of the prepared GHSA-IFNα2b was calculated. The density is 24.
[0048] Sugar density is controlled by the formula:
[0049] Sugar density=4.5+0.12×(IME-thiogalactoside / HSA-IFNα2b)
[0050] In Example 1, GHSA-IFNα2b with different sugar densities can be prepared by controlling the reaction molar ratio of IME-thiogalactoside and HSA-IFNα2b.
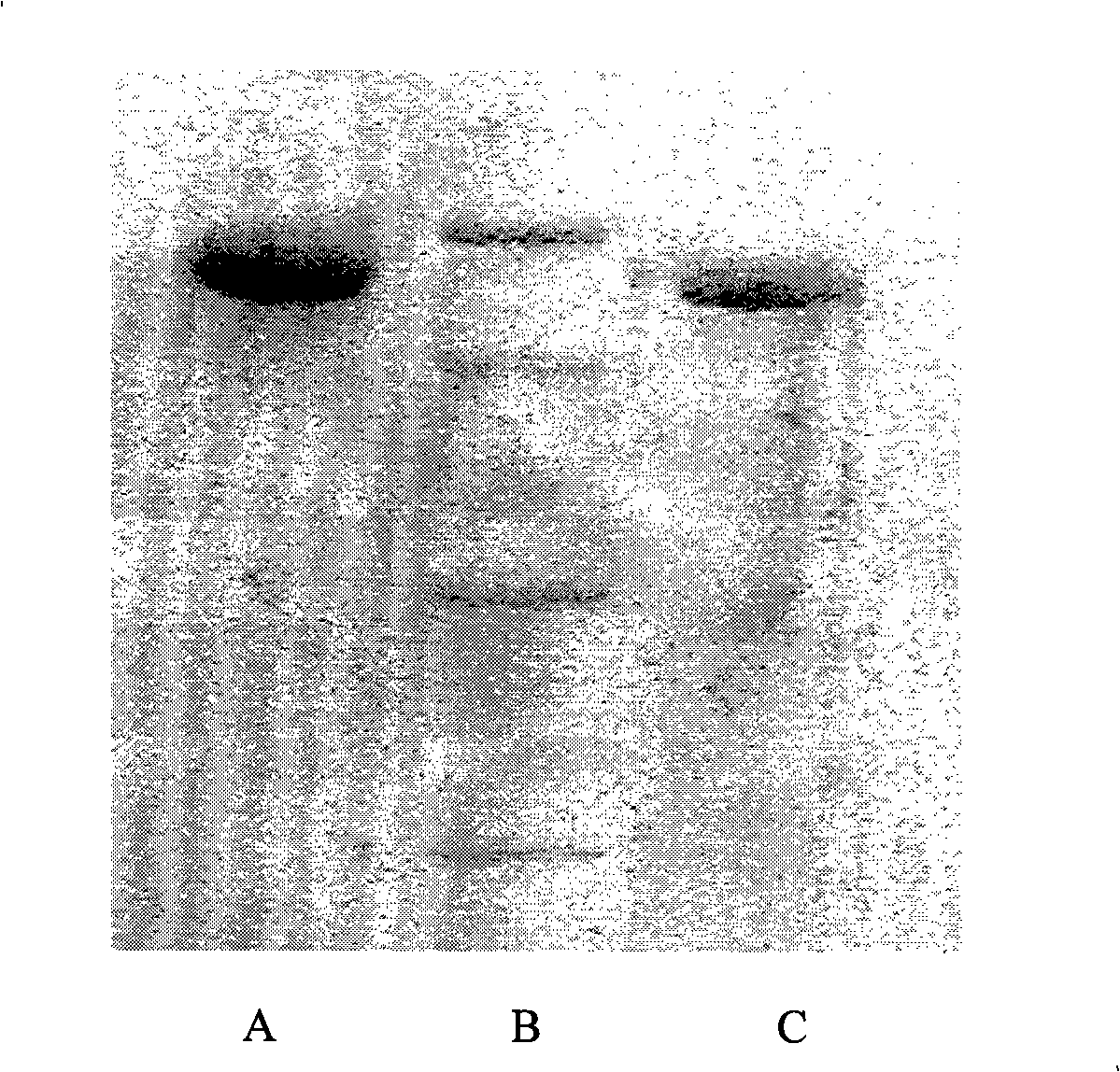
[0051] Proteins with standard molecular weights (attached figure 2 Middle B) and HSA-IFNα2b (attached figure 2 Middle C) is a control, and GHSA-IFNα2b is determined by SDS-PAGE (attached figure 2 The molecular weight in A) shows that half-GHSA-IFNα2b is a single band with a molecular weight ...
Embodiment 3
[0052] Example 3: Analysis of antiviral activity of GHSA-IFNα2b
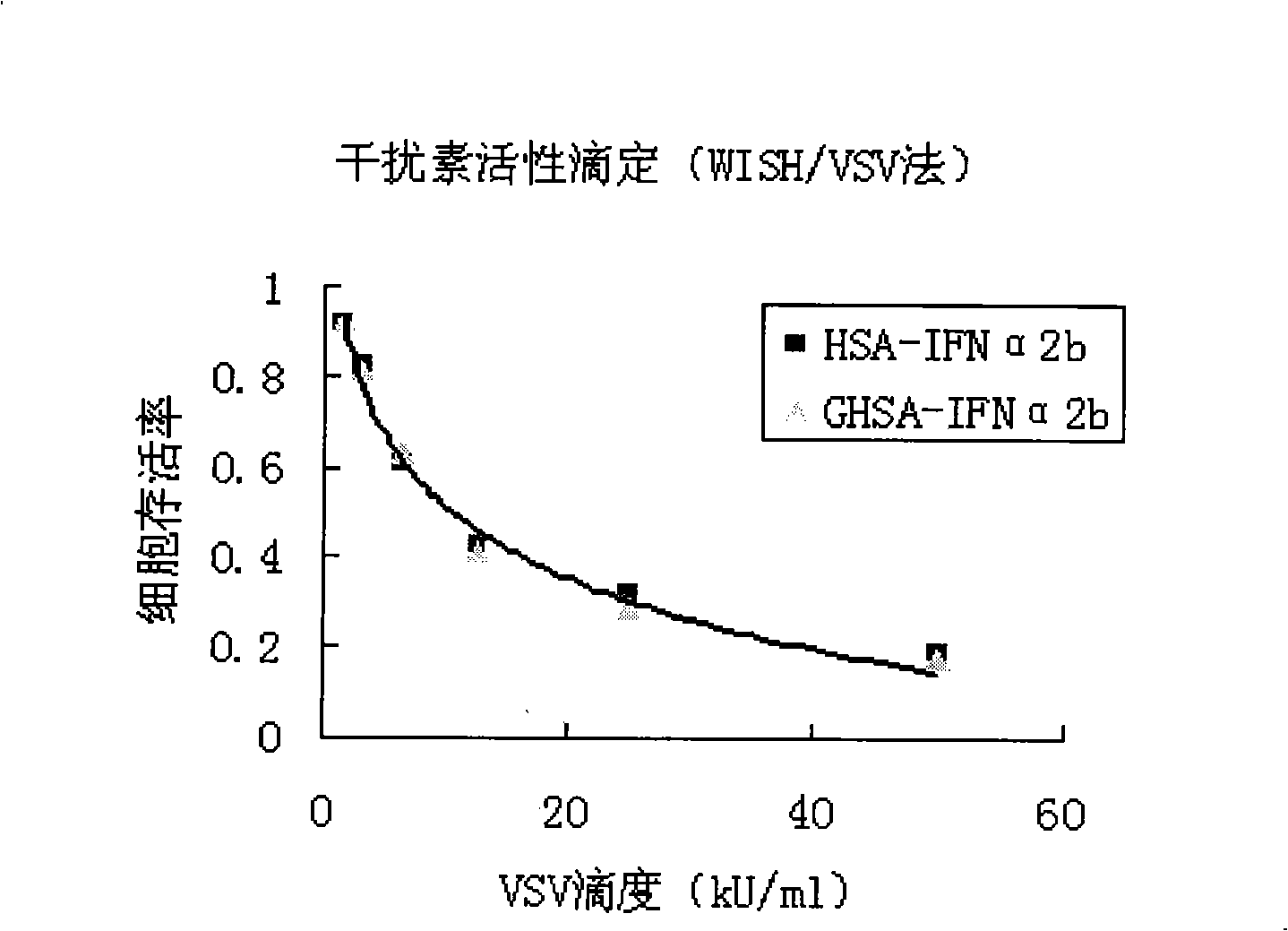
[0053] According to the third appendix XC of Chinese Pharmacopoeia 2005, the biological activity assay of interferon (cytopathic inhibition method), the antiviral activity of HSA-IFN and GHSA-IFNα2b was compared and analyzed by WISH / VSV system, and the results are shown in the appendix image 3 As shown, with the increase of VSV titer, the cell viability gradually decreased. However, the cell survival rates of HSA-IFNα2b and GHSA-IFNα2b corresponding to the same VSV titer were almost the same, which indicated that there was little change in the antiviral activity of HSA-IFNα2b before and after galactosylation.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com