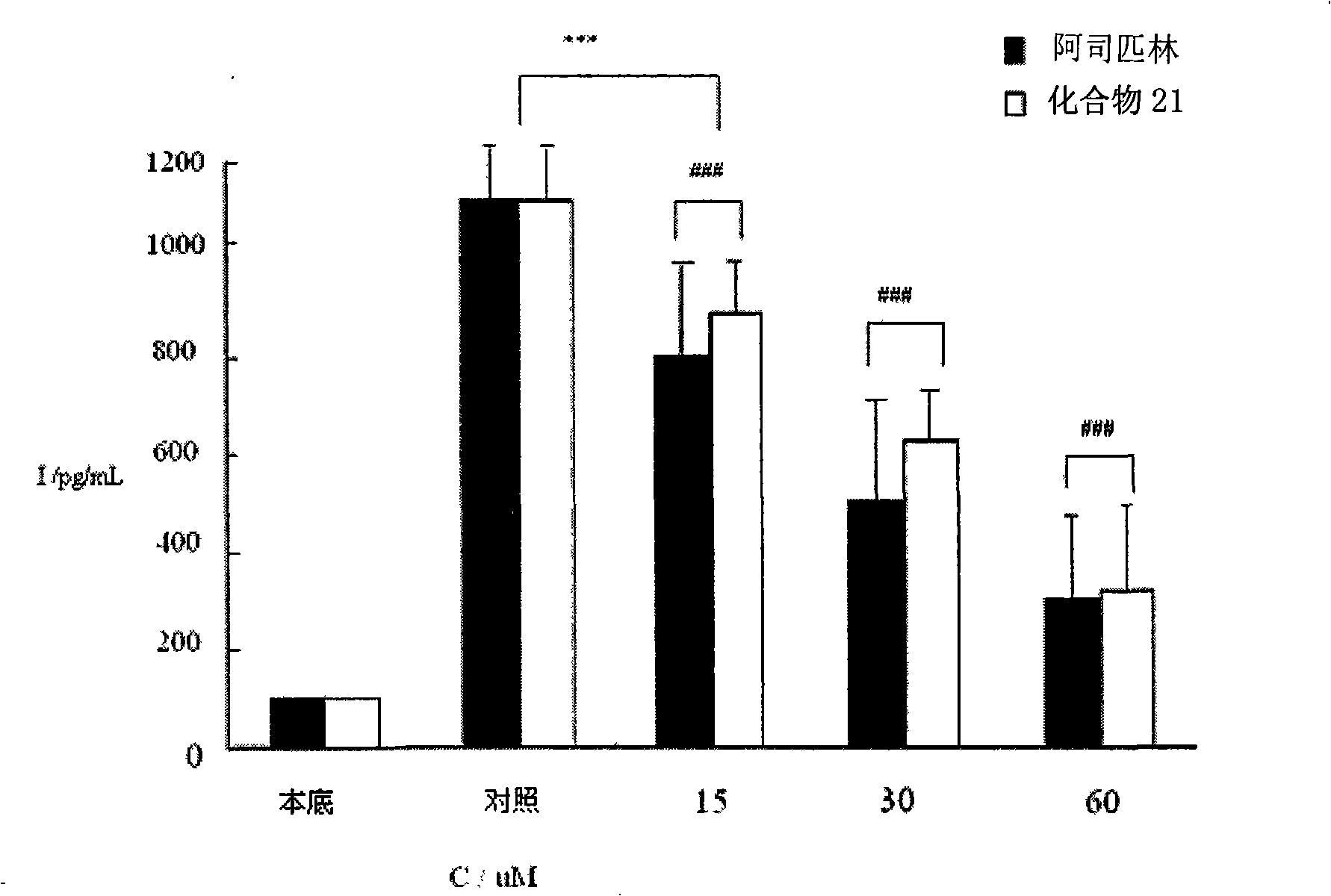
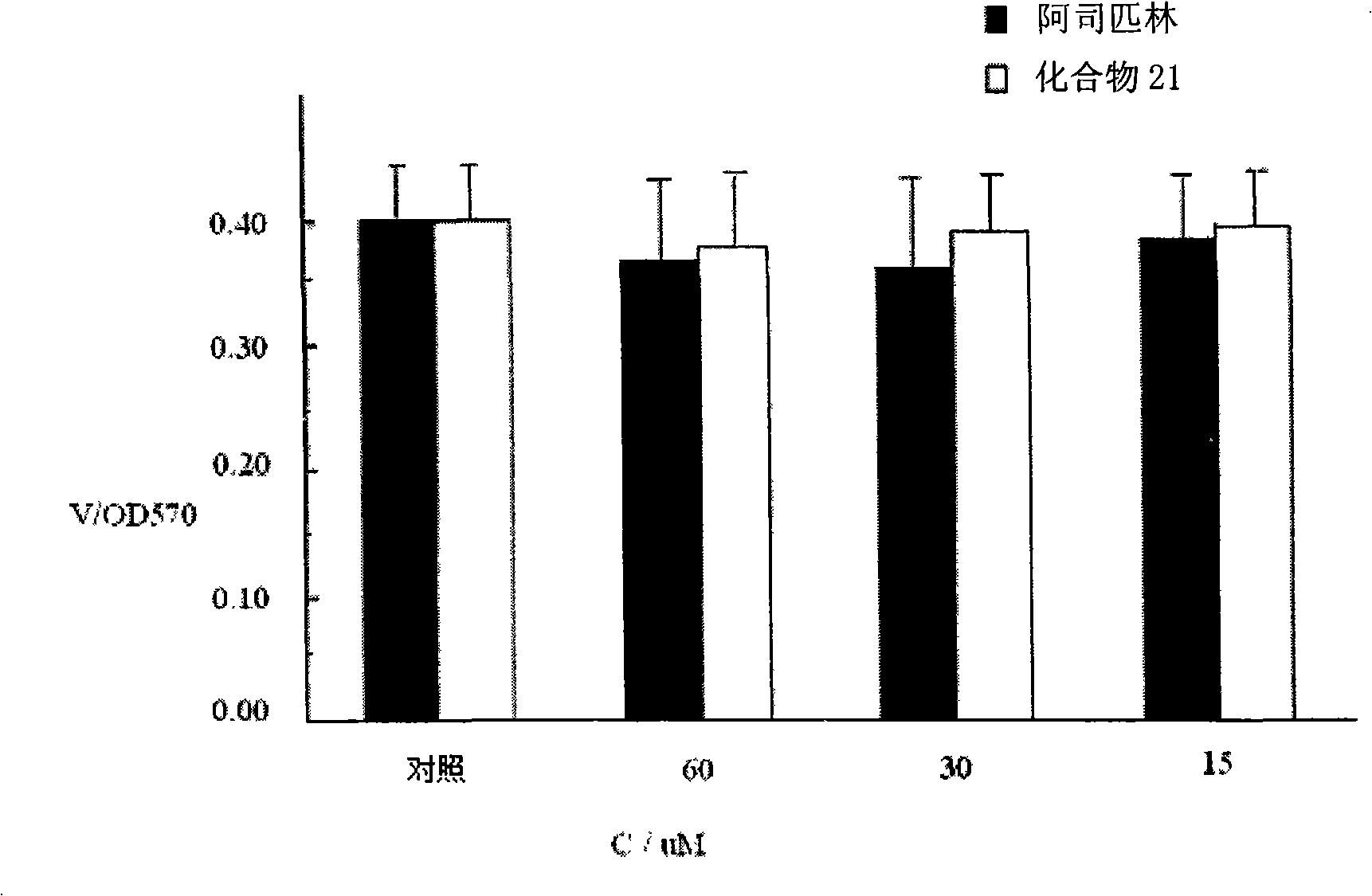
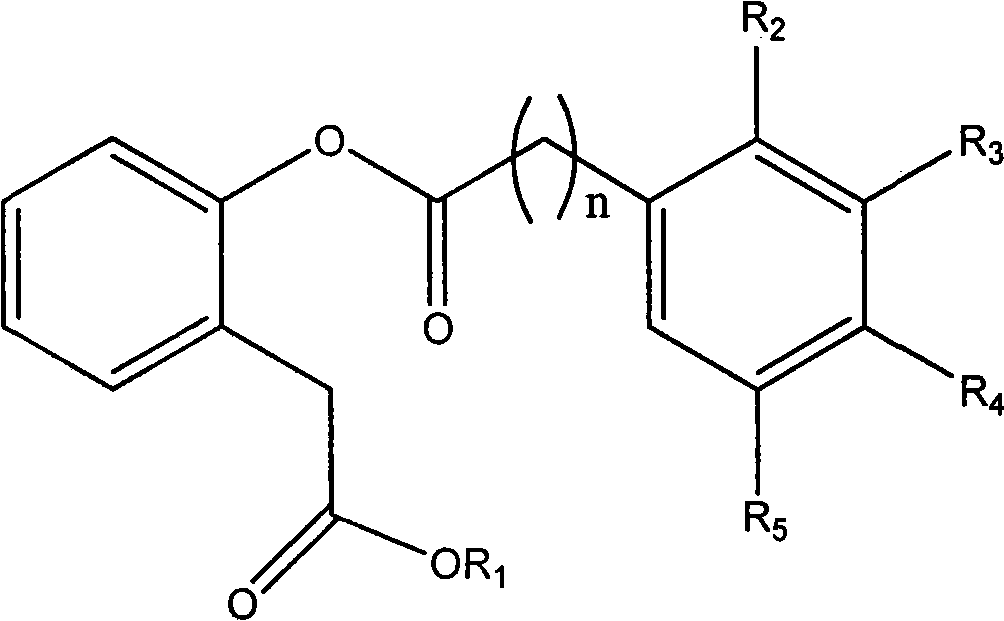
New depside compounds, preparation method thereof and use
A technology of depacids and compounds, which is applied to a new class of depacids and the field of preparation method and application thereof, can solve the problem that there are not many depacids and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Embodiment one: the preparation of methyl o-hydroxyphenylacetate
[0020]
[0021] Dissolve o-hydroxyphenylacetic acid (7.6g, 50mmol) in anhydrous methanol (50mL). After it is completely dissolved, slowly add 3ml of 98% concentrated sulfuric acid dropwise at 90°C under stirring, and stop the reaction after reacting under reflux for 24 hours. . After cooling, add 100mL of water, then extract with ethyl acetate, wash the organic layer twice with saturated brine, then wash with anhydrous Na 2 SO 4 After drying, the solvent was evaporated to dryness under reduced pressure and then separated with a silica gel column. Elution with ethyl acetate:petroleum ether=1:5 gave the target compound. White powder, yield 90%, mp: 61~62℃, 1 H NMR (300MHz, d 6 -DMSO): 3.54 (s, 2H); 3.58 (s, 3H); 6.78 (m, 2H); 7.06 (m, 2H); 9.47 (s, 1H). 13 C NMR (DMSO-d6, δppm): 171.9, 155.5, 131.2, 128.2, 121.4, 118.9, 115.0, 51.6, 35.2. MS (ESI): 167.1 (C 9 h 11 o 3 ,[M+H] + ).Anal.Calcd for...
Embodiment 2
[0022] Embodiment two: the preparation of ethyl o-hydroxyphenylacetate
[0023]
[0024] Dissolve o-hydroxyphenylacetic acid (7.6g, 50mmol) in absolute ethanol (50mL), and after it is completely dissolved, slowly add 3.5ml of 98% concentrated sulfuric acid dropwise under stirring at 95°C, and stop the reaction after 24 hours under reflux reaction. After cooling, add 100mL of water, then extract with ethyl acetate, wash the organic layer twice with saturated brine, then wash with anhydrous Na 2 SO 4 After drying, the solvent was evaporated to dryness under reduced pressure, separated by silica gel column, and eluted with ethyl acetate:petroleum ether=1:4.5 to obtain the target compound as a white powder. Yield 86%, mp: 67~68℃; 1 H NMR (300MHz, d 6 -DMSO): 1.17 (t, J = 7.1 Hz, 3H); 3.53 (s, 2H); 4.01 (m, 2H); 6.75 (m, 2H); 7.07 (m, 2H); 9.46 (s, 1H) . 13 C NMR (DMSO-d6, δppm): 171.6, 155.3, 131.8, 127.9, 121.8, 118.3, 115.4, 51.5, 35.8, 14.3. MS (ESI): 181.1 (C 10 h 1...
Embodiment 3
[0025] Example 3: Preparation of 4-fluorophenylacetic acid-2-(methoxycarbonylmethyl)phenol ester (compound 1).
[0026]
[0027] The product methyl o-hydroxyphenylacetate (1.66g, 10mmol) obtained in Example 1 was dissolved in anhydrous dichloromethane (50mL), and after being completely dissolved, 4-fluorophenylacetic acid ( 1.85 g, 12 mmol). Then add N,N-dimethylaminopyridine (DMAP) (248mg, 2.03mmol) and N,N-dicyclohexylcarbodiimide (DCC) (2.26g, 11mmol), and stir under reflux for 12 hours to stop the reaction . After being completely cooled, add 100mL of water, then extract with ethyl acetate, wash the organic layer twice with saturated brine, and then wash with anhydrous Na 2 SO 4 After drying, the solvent was evaporated to dryness under reduced pressure and then separated with a silica gel column, eluting with ethyl acetate:petroleum ether=1:5 to obtain the target compound as a light yellow oil. Yield 89%, 1 HNMR (300MHz, d 6 -DMSO): 3.54 (s, 2H); 3.55 (s, 3H); 3.95 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com