Method for synthesizing 1,3,5-triamino-2,4,6-trinitrobenzene
A technology of trinitrobenzene and trinitroaniline, applied in the field of material chemistry, can solve the problems of complex reaction operation, toxic reactants, harsh reaction conditions, etc., and achieve the effects of easy-to-obtain raw materials, short reaction time, and convenient post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
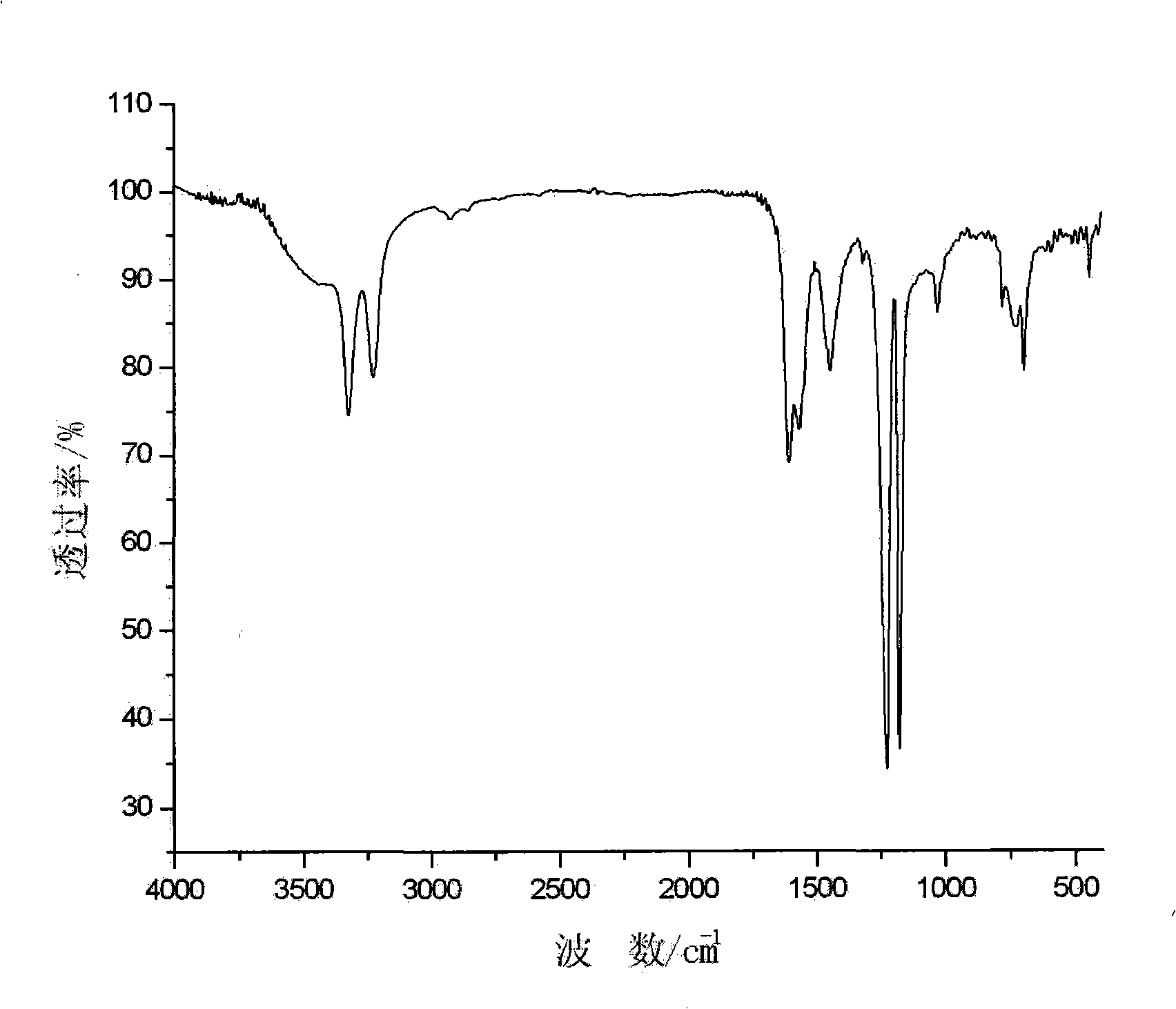
[0022] In a 100ml reaction vessel, dissolve 1.312g of hydroxylamine sulfate, 0.457g of TNA, and 3.808g of sodium ethoxide in a mixed solvent of 50ml of DMSO and toluene, stir evenly, then heat up to 70°C, and react for 8 hours. After the reaction is complete, cool, pour into ice water, filter, wash with acetone and distilled water respectively, and dry in a vacuum oven to obtain tan powder, which is the target product TATB, with a yield of 62.4%. The infrared spectrum of the product is as figure 1 As shown, the mass spectrogram is shown in Figure 2.
Embodiment 2
[0024] In a 100ml reaction vessel, dissolve 0.834g of hydroxylamine hydrochloride, 0.457g of TNA, and 2.056g of sodium ethoxide in 50ml of DMSO, stir evenly, heat up to 80°C, and react for 10 hours. After the reaction is complete, cool, pour into acetic acid aqueous solution, filter, wash with acetone and distilled water respectively, and dry in a vacuum oven to obtain tan powder which is the target product TATB, yield: 70.2%.
Embodiment 3
[0026] In a 100ml reaction vessel, dissolve 0.985g of hydroxylamine hydrochloride, 0.222g of TNA, and 2.592g of sodium methoxide in 25ml of DMSO, stir evenly, raise the temperature to 80°C, and react for 12 hours. After the reaction is complete, cool down, pour into 200ml hydrochloric acid aqueous solution, filter, rinse with acetone and distilled water respectively, and dry in a vacuum oven to obtain yellow TATB with a yield of 77.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com