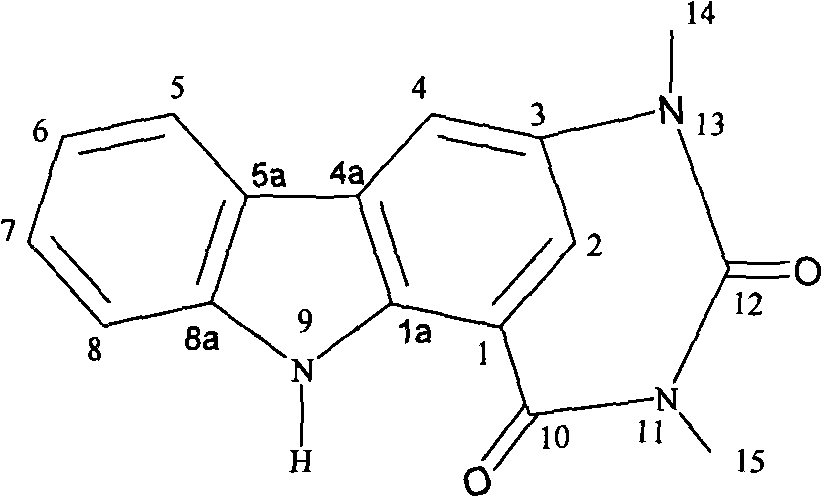
Carbazoles alkaloid, preparation and application thereof
A compound and crude product technology, used in carbazole alkaloids, the application of inhibiting cancer cell growth and anti-virus, the field of preparation of alkaloids
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Using antipathes dichotcma (Pallas) with a wet weight of 3Kg as raw material, freeze-dried and chopped, extracted 3 times with 15 L of ethanol-dichloromethane mixed organic solvent with a volume ratio of 2:1, and decompressed the extract Concentrated crude extract. The crude extract was suspended and dissolved in water, extracted three times with ethyl acetate (500 mL each time), and the ethyl acetate layer was taken and concentrated under reduced pressure to obtain 27 g. Use chloroform-methanol mixed solvent to dissolve the ethyl acetate layer and mix with silica gel, mix about 600mL petroleum ether with approximately 500g silica gel for wet packing, and change the volume ratio of petroleum ether-ethyl acetate solvent system from 10:0 to 0:10 Carry out gradient elution, thin-layer chromatography TLC (GF 254 ) to track and merge 8 components. These 8 components were tested in TLC (GF 254 ) was washed with chloroform-acetone solvent system from 10:1 to 9:1 by volume r...
Embodiment 2
[0023] The logarithmic growth phase SGC-7901 and Hep_G2 cell lines were collected and seeded in 96-well culture plates respectively, with the number of cells per well being 1.0×10 5 / 100μL in 5% CO 2 Cultivate in an incubator, remove the medium the next day, add 100 μL of drugs of different concentrations (the drug concentration is double-diluted), no drug is added to the negative control group, after 48 hours, add 10 μL of MTT to each well, continue to cultivate for 4 hours, and then add 100 μL of DMSO to each well Terminate the reaction, place it at room temperature for 1h, detect the absorbance A value of each well at 570nm with a microplate reader, and calculate the cell growth inhibition rate. The calculation formula is: growth inhibition rate (%)=[(A 阴性 -A 实验 ) / (A 阴性 -A 空白 )] × 100%. The half inhibitory rate IC50 of SGC-7901 and Hep_G2 cell growth were 67.38μg / mL and 73.65μg / mL respectively.
Embodiment 3
[0025] Collect vero cells in the logarithmic growth phase and inoculate them in 96-well culture plates, the number of cells per well is 1.0×10 5 / 100μL, placed in 5%, CO 2 Incubator cultivation. The next day, suck out the growth solution in the microwells, add 50 μL of the diluted solution of the compound of formula (1) in Example 1 to each well, immediately add 50 μL of HSV-1 virus use solution, and set normal cell control and virus control at the same time, 37 ° C , 5% CO 2 Incubator cultivation. Observe the cytopathic effect (CPE), observe the cell growth every day, and express the cytopathic condition by -, +, ++, +++, ++++. The results show that the compound of the present invention has a maximum nontoxic concentration of 25ug / ml to vero cells, and it inhibits the IC of herpes simplex virus type I lesions. 50 The value was 30.68ug / mL.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com