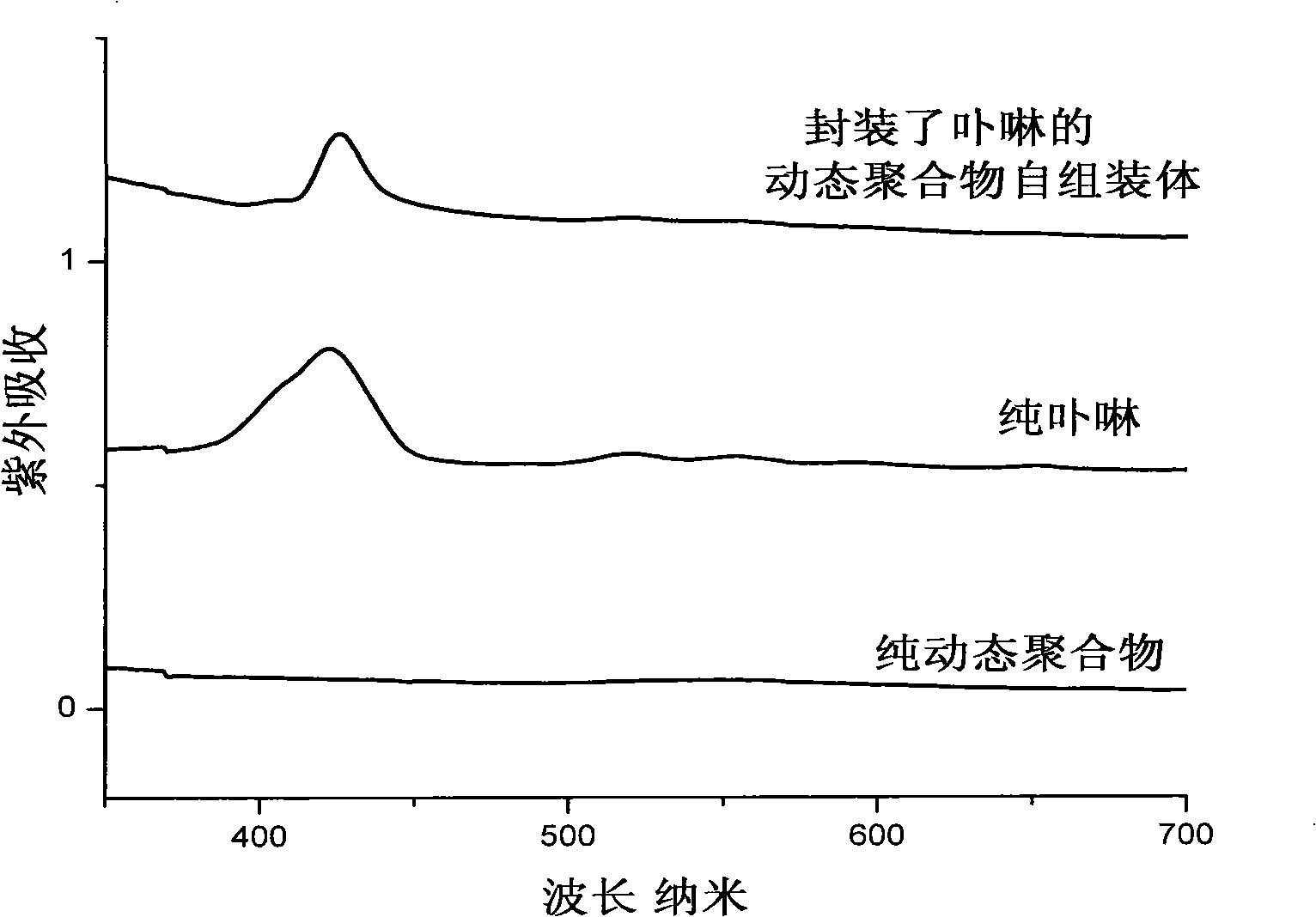
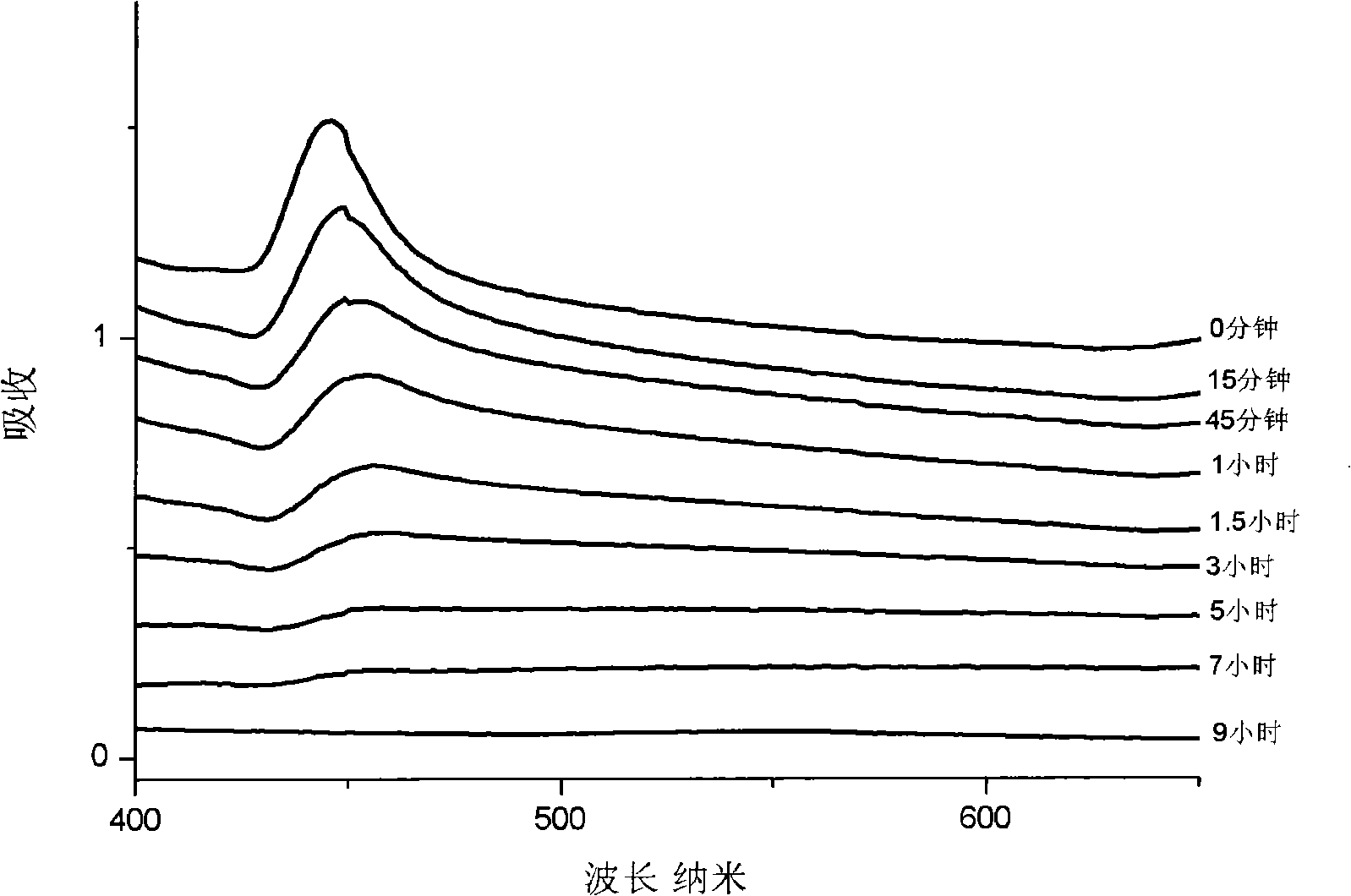
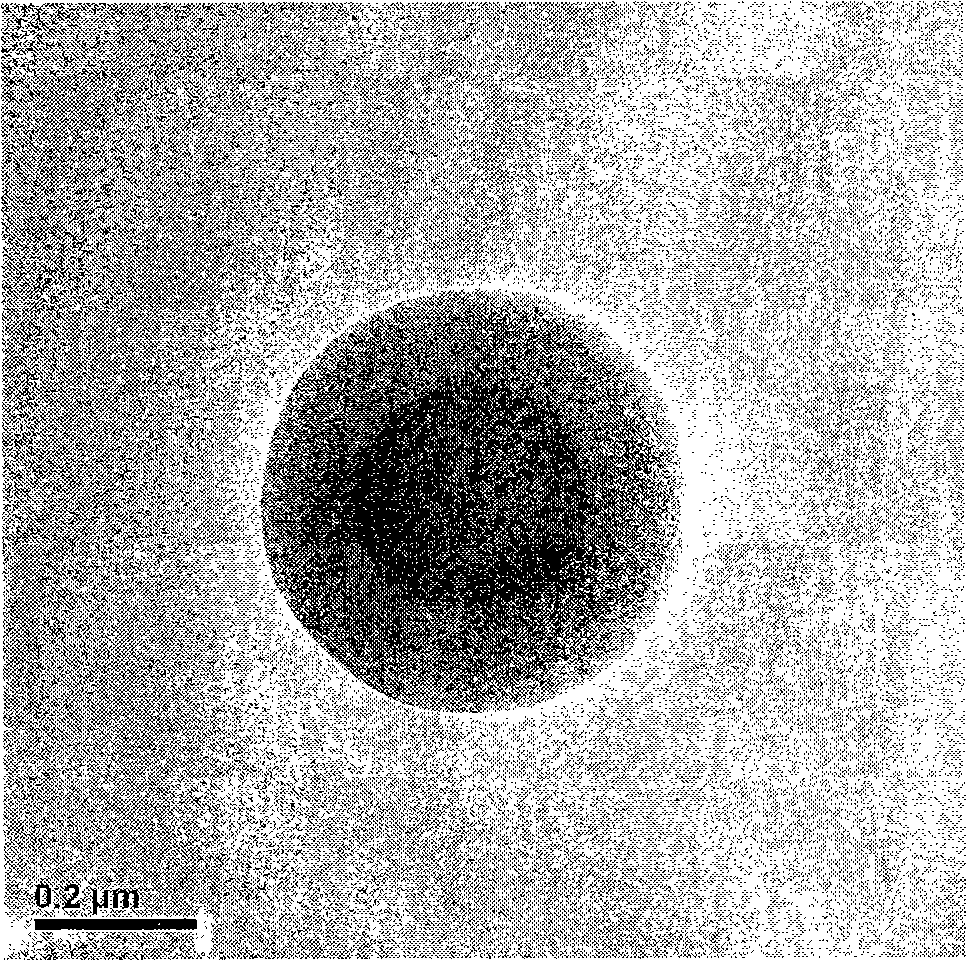Preparation method for pH controlled releasedynamic polymer packaging system
A controlled release and polymer technology, which is applied in the direction of medical preparations with non-active ingredients, medical preparations containing active ingredients, drug combinations, etc., can solve the problem of abnormal rapidity, uncontrollable release, and difficulty in dissociation, etc. problems, to achieve the effect of broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Step 1: Add 7 grams of styrene monomer, 1.25 grams of benzyl bromide, 0.5 grams of cuprous bromide and 2 grams of bipyridyl into a 50-ml flask, bubbling nitrogen gas for 30 minutes, sealing it, and putting it in 110°C oil In the bath, react for 3 hours. Add a small amount of tetrahydrofuran to dissolve it, throw it into a large amount of methanol for precipitation twice, and dry it in a vacuum oven at 50°C for 1 day to obtain polystyrene with a molecular weight of 2,000.
[0034] Step 2: Add 2 grams of anhydrous potassium carbonate and 1 gram of p-hydroxybenzaldehyde to the acetone solution of 3 grams of polystyrene obtained above, and reflux at 60° C. for 1 day. By filtering, concentrating the solution, precipitating a large amount of methanol three times, and drying in vacuum at 50° C., polystyrene with an aldehyde group at the end can be obtained.
[0035] Step 3: Dissolve 20 mg of the above polystyrene and 10 mg of hydrazide-terminated monomethyl polyethylene glyco...
Embodiment 2
[0039] Step 1: Add 7 grams of styrene monomer, 1.25 grams of benzyl bromide, 0.5 grams of cuprous bromide and 2 grams of bipyridyl into a 50-ml flask, bubbling nitrogen gas for 30 minutes, sealing it, and putting it in 110°C oil In the bath, react for 3 hours. Add a small amount of tetrahydrofuran to dissolve it, throw it into a large amount of methanol for precipitation twice, and dry it in a vacuum oven at 50°C for 1 day to obtain polystyrene with a molecular weight of 2,000.
[0040] Step 2: Add 2 grams of anhydrous potassium carbonate and 1 gram of p-hydroxybenzaldehyde to the acetone solution of 3 grams of polystyrene obtained above, and reflux at 60° C. for 1 day. By filtering, concentrating the solution, precipitating a large amount of methanol, and drying in vacuum at 50°C, polystyrene with aldehyde groups at the end can be obtained.
[0041] The third step: 20 mg of the above-mentioned polystyrene and 5 mg of monomethyl polyethylene glycol (molecular weight: 550) wit...
Embodiment 3
[0045] Step 1: Add 7 grams of styrene monomer, 0.5 grams of benzyl bromide, 0.5 grams of cuprous bromide and 2 grams of bipyridyl into a 50-ml flask, bubbling nitrogen gas for 30 minutes, sealing it, and putting it in 110°C oil In the bath, react for 3 hours. Add a small amount of tetrahydrofuran to dissolve it, throw it into a large amount of methanol for precipitation twice, and dry it in a vacuum oven at 50°C for 1 day to obtain polystyrene with a molecular weight of 3000.
[0046] Step 2: Add 2 g of anhydrous potassium carbonate and 0.8 g of p-hydroxybenzaldehyde to the acetone solution of 3 g of polystyrene obtained above, and reflux at 60° C. for 1 day. By filtering, concentrating the solution, precipitating a large amount of methanol, and drying in vacuum at 50°C, polystyrene with aldehyde groups at the end can be obtained.
[0047] Step 3: Dissolve 15 mg of the above polystyrene and 10 mg of monomethyl polyethylene glycol (molecular weight 2000) with a hydrazide bond ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com