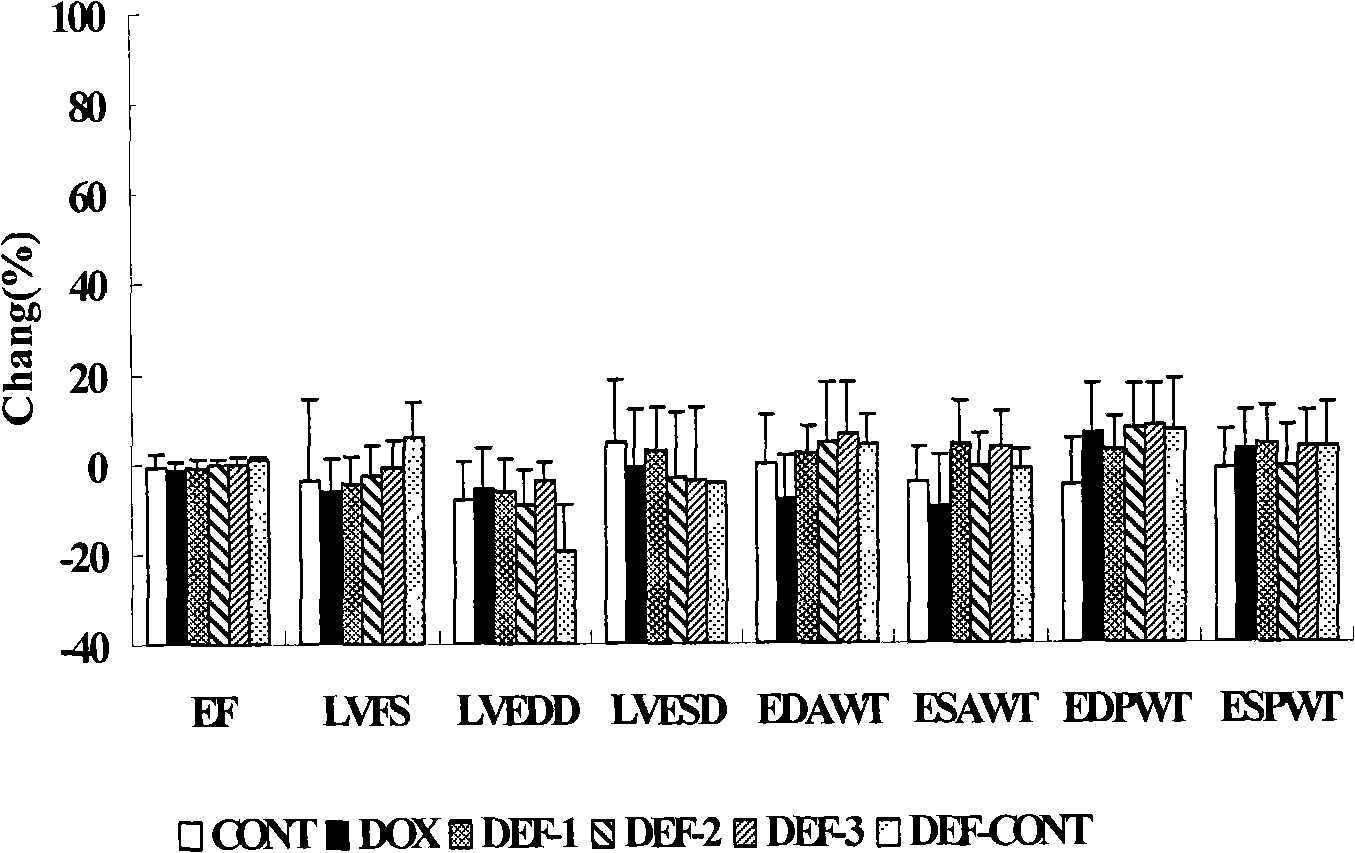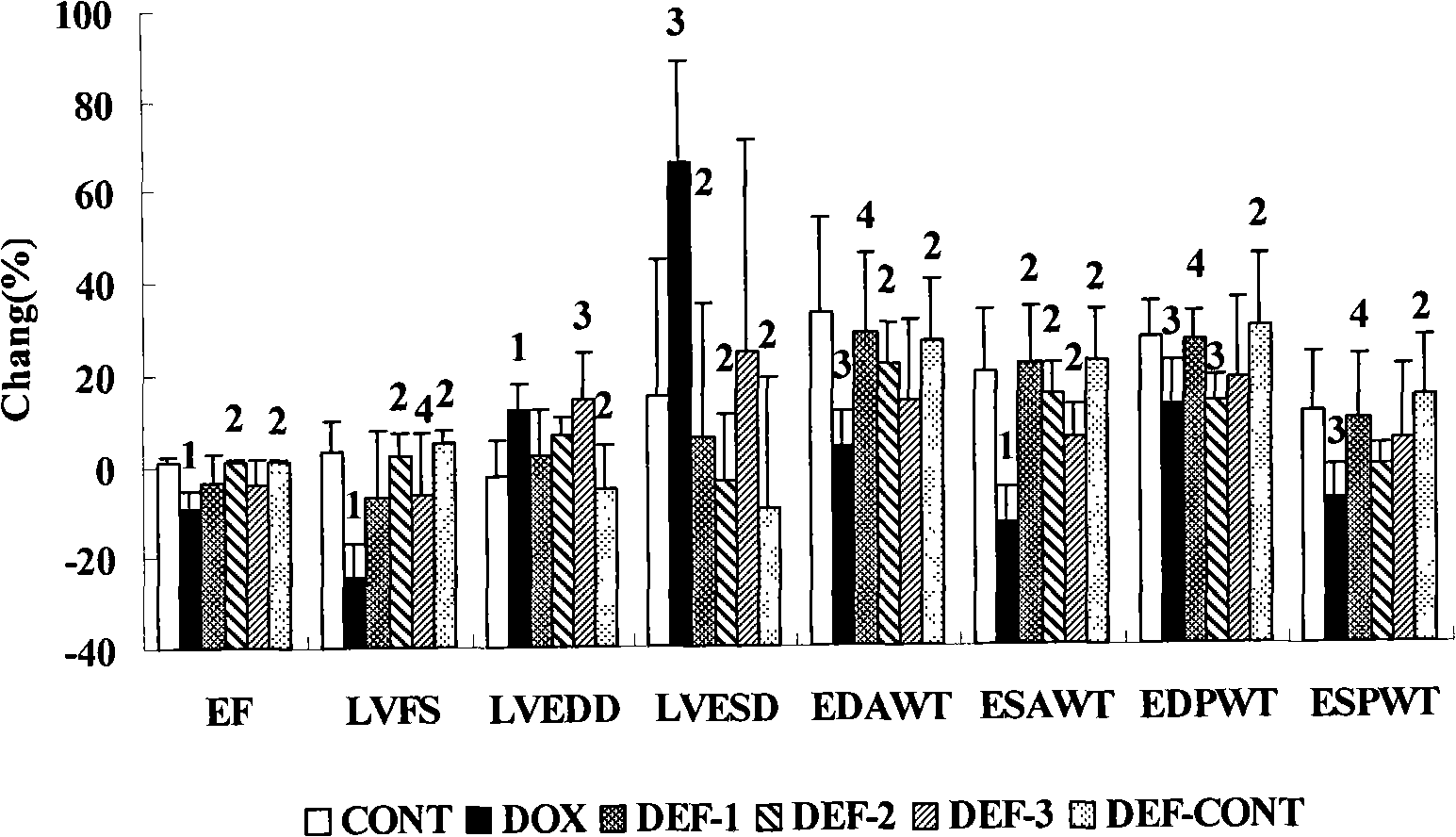Uses of deferiprone and formulation thereof in preparing medicament for preventing and treating cardiotoxicity induced by anthracyclines
A technology of cardiotoxicity and anthracyclines, which is applied in the field of medicine, can solve the problems of no reports on the development of deferiprone cardioprotective agent preparations, etc., and achieve the effects of reducing myocardial peroxidative damage, improving therapeutic index, and reducing cardiotoxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
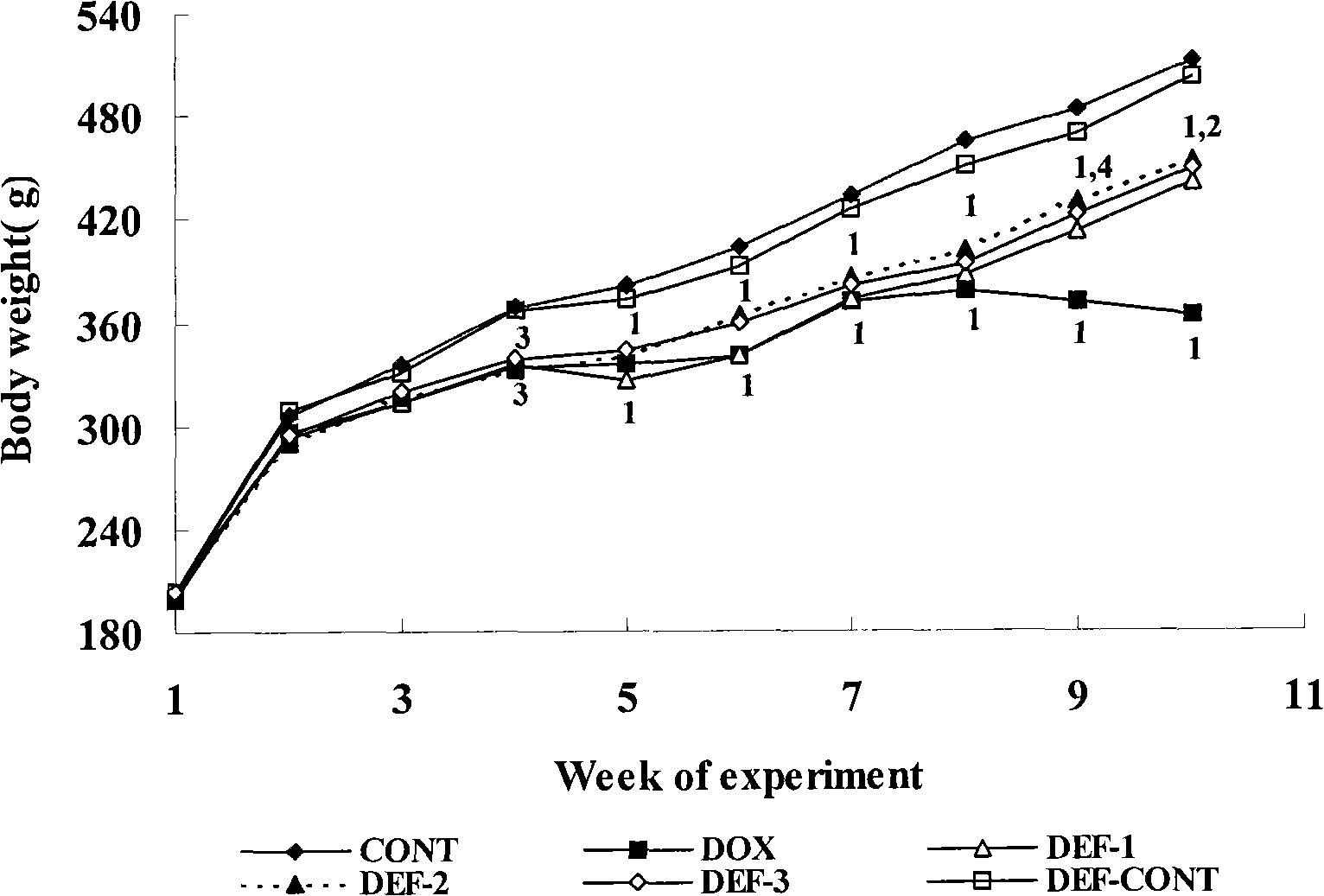
[0056] Example 1 Deferiprone (Deferiprone) prevents and treats cardiotoxicity in rats caused by doxorubicin (DOX)
[0057] 1.1 Establishment of doxorubicin heart failure model and dose setting of deferiprone The experiment lasted for 10 weeks, the drug was stopped at the 8th week, and the observation continued for 2 weeks. Get 72 rats, divide into 6 groups at random according to body weight, administer according to the following method:
[0058] Adriamycin group: intraperitoneal injection of 2.5 mg / kg (4.31 μmol / kg) of adriamycin, once a week, for 8 consecutive weeks.
[0059] Blank control group: intraperitoneal injection of equal volume of normal saline, 5 days a week, for 8 consecutive weeks.
[0060] Deferiprone treatment group: Different doses of deferiprone were administered 5 days a week for 8 consecutive weeks. in:
[0061] Deferiprone group 1: the treatment was the same as that of the doxorubicin group, and a low dose of deferiprone 1.71 mg / kg was given intraperito...
Embodiment 2
[0103] Accurately weigh deferiprone, lactic acid-glycolic acid copolymer (PLGA) and / or polylactic acid (PLA) according to a certain proportion, dissolve them in an appropriate amount of dichloromethane, put them in an oil bath at 37°C, and evaporate the solvent to make a self-made extruder Extrusion. After drying in a vacuum oven at room temperature to constant weight, it was cut into small rods with a diameter×length of 2mm×20mm, and the average weight of the preparation was 84.23±11.01mg to obtain the implant of the present invention.
Embodiment 3
[0105] Prevention and treatment of deferiprone biodegradable implants on cardiotoxicity induced by doxorubicin in rats
[0106] 3.1 Grouping and administration
[0107] The experiment was divided into 4 groups and lasted 30 days. Table 3 is the dosage regimen of each group.
[0108] table 3
[0109]
[0110] After administration, the general situation was observed, and the changes in body weight and survival rate were recorded. Animals that died during the experiment were weighed and dissected in time. The experiment was ended on the 30th day after administration, and the ascites volume and liver and kidney edema were recorded after the animals were sacrificed by bloodletting; the left ventricular muscle at the apex of the heart was fixed with 10% formaldehyde for pathological examination.
[0111] 3.2 Effect of deferiprone implant on the survival rate of doxorubicin-administered rats
[0112] After the implantation operation, there was no redness, swelling and exudati...
PUM
| Property | Measurement | Unit |
|---|---|---|
| solubility (mass) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com